Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model
- PMID: 18385079
- PMCID: PMC2362384
- DOI: 10.1167/iovs.07-1356
Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model
Abstract
Purpose: To study NAD(P)H oxidase-dependent outcomes after oxygen stresses that are similar to those experienced by preterm infants today using a rat model of retinopathy of prematurity.
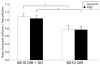
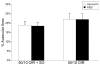
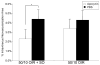
Methods: Within 4 hours of birth, pups and their mothers were cycled between 50% and 10% oxygen daily for 14 days and were returned to room air (21% O2, 50/10 oxygen-induced retinopathy [OIR]) or supplemental oxygen (28% O2, 50/10 OIR+SO) for 4 days. Pups received intraperitoneal injections of the specific NAD(P)H oxidase inhibitor apocynin (10 mg/kg/d) or of PBS from postnatal day (P)12 to P17, and some received intraperitoneal injections of hypoxyprobe before kill. Intravitreous neovascularization (IVNV), avascular/total retinal areas, vascular endothelial growth factor (VEGF), NAD(P)H oxidase activity, or hypoxic retina (conjugated hypoxyprobe) were determined in neurosensory retinas. Human retinal microvascular endothelial cells (RMVECs) treated with apocynin or control were exposed to 1% or 21% O2 and assayed for phosphorylated (p-)Janus kinase (JNK) and NAD(P)H oxidase activity.
Results: Retinas from 50/10 OIR+SO had increased NAD(P)H oxidase activity and lower VEGF than did retinas from 50/10 OIR. Apocynin treatment reduced the IVNV area and hypoxic retina in 50/10 OIR+SO. RMVECs treated with 1% O2 had increased p-JNK compared with RMVECs exposed to room air.
Conclusions: Different oxygen stresses activate NAD(P)H oxidase to varying degrees to trigger disparate pathways (angiogenesis or apoptosis). The oxygen stresses and outcomes used in this study are relevant to human ROP and may explain some of the complexity in the pathophysiology of ROP resulting from oxygen exposure.
Figures

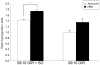



Similar articles
-
Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity.Mol Vis. 2007 Jun 12;13:840-53. Mol Vis. 2007. PMID: 17615545 Free PMC article.
-
The role of supplemental oxygen and JAK/STAT signaling in intravitreous neovascularization in a ROP rat model.Invest Ophthalmol Vis Sci. 2009 Jul;50(7):3360-5. doi: 10.1167/iovs.08-3256. Epub 2009 Mar 5. Invest Ophthalmol Vis Sci. 2009. PMID: 19264880 Free PMC article.
-
Endothelial NADPH oxidase 4 mediates vascular endothelial growth factor receptor 2-induced intravitreal neovascularization in a rat model of retinopathy of prematurity.Mol Vis. 2014 Mar 3;20:231-41. eCollection 2014. Mol Vis. 2014. PMID: 24623966 Free PMC article.
-
The effects of oxygen stresses on the development of features of severe retinopathy of prematurity: knowledge from the 50/10 OIR model.Doc Ophthalmol. 2010 Feb;120(1):25-39. doi: 10.1007/s10633-009-9181-x. Epub 2009 Jul 29. Doc Ophthalmol. 2010. PMID: 19639355 Free PMC article. Review.
-
Studies on the pathogenesis of avascular retina and neovascularization into the vitreous in peripheral severe retinopathy of prematurity (an american ophthalmological society thesis).Trans Am Ophthalmol Soc. 2010 Dec;108:96-119. Trans Am Ophthalmol Soc. 2010. PMID: 21212851 Free PMC article. Review.
Cited by
-
Signaling pathways triggered by oxidative stress that mediate features of severe retinopathy of prematurity.JAMA Ophthalmol. 2013 Jan;131(1):80-5. doi: 10.1001/jamaophthalmol.2013.986. JAMA Ophthalmol. 2013. PMID: 23307212 Free PMC article.
-
Anti-VEGF antibody leads to later atypical intravitreous neovascularization and activation of angiogenic pathways in a rat model of retinopathy of prematurity.Invest Ophthalmol Vis Sci. 2013 Mar 21;54(3):2020-6. doi: 10.1167/iovs.13-11625. Invest Ophthalmol Vis Sci. 2013. PMID: 23449716 Free PMC article.
-
Docosahexaenoic acid improves the nitroso-redox balance and reduces VEGF-mediated angiogenic signaling in microvascular endothelial cells.Invest Ophthalmol Vis Sci. 2010 Dec;51(12):6815-25. doi: 10.1167/iovs.10-5339. Epub 2010 Aug 11. Invest Ophthalmol Vis Sci. 2010. PMID: 20702831 Free PMC article.
-
Roles of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase in Angiogenesis: Isoform-Specific Effects.Antioxidants (Basel). 2017 Jun 3;6(2):40. doi: 10.3390/antiox6020040. Antioxidants (Basel). 2017. PMID: 28587189 Free PMC article. Review.
-
Potential Effects of Nutraceuticals in Retinopathy of Prematurity.Life (Basel). 2021 Jan 22;11(2):79. doi: 10.3390/life11020079. Life (Basel). 2021. PMID: 33499180 Free PMC article. Review.
References
-
- Patz A, Eastham A, Higginbotham DH, Kleh T. Oxygen studies in retrolental fibroplasia. Am J Ophthalmol. 1953;36:1511–1522. - PubMed
-
- Gao G, Li Y, Zhang D, et al. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett. 2001;489:270–276. - PubMed
-
- Chan-Ling T, Gock B, Stone J. Supplemental oxygen therapy: basis for noninvasive treatment of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 1995;36:1215–1230. - PubMed
-
- Smith LEH, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

