Elevated MMP Expression in the MRL Mouse Retina Creates a Permissive Environment for Retinal Regeneration
- PMID: 18385092
- PMCID: PMC2613950
- DOI: 10.1167/iovs.07-1058
Elevated MMP Expression in the MRL Mouse Retina Creates a Permissive Environment for Retinal Regeneration
Abstract
Purpose: The MRL/MpJ (healer) mouse is an established model for autoimmune studies and was recently identified as having a profound ability to undergo scarless regeneration of the tissue in the ear and heart. This regenerative capacity has been linked to elevated matrix metalloproteinase (MMP)-2 and -9 expression, giving this mouse the ability to degrade and remove inhibitory basement membrane molecules. Although elevated MMP expression has been reported in somatic tissues in this strain, little is known about MMP expression and the response to injury in the MRL/MpJ mouse retina. The purpose of this study was to investigate whether increased MMP expression and subsequent decreased inhibitory extracellular matrix molecule deposition in the MRL/MpJ mouse retina produces a permissive regenerative environment.
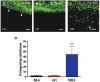
Methods: Experiments were performed using 3- to 4-week-old MRL/MpJ, retinal degenerative (rd1), and C57BL/6 (wild-type) mice. Western blotting, oligo-microarray, and immunohistochemical analyses were used to determine the level and location of MMP and extracellular matrix (ECM) protein expression. Retinal responses to injury were modeled by retinal detachment in vivo and in retinal explantation in vitro. The capacity of the retinal environment to support photoreceptor cell migration, integration, or regeneration was analyzed using hematoxylin-eosin, immunohistochemical staining, and cell counting.
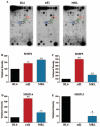
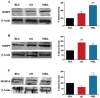
Results: Compared with C57BL/6J animals, MRL/MpJ mice exhibit elevated levels of MMP-2, -9, and -14 and decreased levels of the inhibitory proteins neurocan and CD44 within the retina. Although similar increases in MMP-2, -9, and CD44s (CD44 degradation product) were observed in the rd1 retina, elevated levels of the inhibitory ECM molecules (neurocan and CD44) remained. Thus, the MRL retinal environment, which expresses lower levels of inhibitory ECM molecules after injury, was more conducive to regeneration and enhanced photoreceptor integration in vitro than C57BL/6J or rd1 controls.
Conclusions: The MRL mouse retina shows elevated MMP expression and decreased levels of scar-related inhibitory molecules, which leads to a retinal environment that is more permissive for neural regeneration and cell integration after in vitro retinal explantation.
Figures



Similar articles
-
Laser injury promotes migration and integration of retinal progenitor cells into host retina.Mol Vis. 2010 Jun 4;16:983-90. Mol Vis. 2010. PMID: 20577598 Free PMC article.
-
Uncorrelated healing response of tendon and ear injuries in MRL highlight a role for the local tendon environment in driving scarless healing.Connect Tissue Res. 2018 Sep;59(5):472-482. doi: 10.1080/03008207.2018.1485665. Epub 2018 Jun 21. Connect Tissue Res. 2018. PMID: 29929396 Free PMC article.
-
Regenerative MRL/MpJ tendon cells exhibit sex differences in morphology, proliferation, mechanosensitivity, and cell-ECM organization.J Orthop Res. 2023 Oct;41(10):2273-2286. doi: 10.1002/jor.25562. Epub 2023 Apr 11. J Orthop Res. 2023. PMID: 37004178
-
Spallanzani's mouse: a model of restoration and regeneration.Curr Top Microbiol Immunol. 2004;280:165-89. doi: 10.1007/978-3-642-18846-6_5. Curr Top Microbiol Immunol. 2004. PMID: 14594211 Review.
-
The scarless heart and the MRL mouse.Philos Trans R Soc Lond B Biol Sci. 2004 May 29;359(1445):785-93. doi: 10.1098/rstb.2004.1468. Philos Trans R Soc Lond B Biol Sci. 2004. PMID: 15293806 Free PMC article. Review.
Cited by
-
Antiangiogenic cytokines as potential new therapeutic targets for resveratrol in diabetic retinopathy.Drug Des Devel Ther. 2018 Jul 3;12:1985-1996. doi: 10.2147/DDDT.S156941. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30013318 Free PMC article. Review.
-
Extracellular, stem cells and regenerative ophthalmology.J Glaucoma. 2014 Oct-Nov;23(8 Suppl 1):S30-3. doi: 10.1097/IJG.0000000000000112. J Glaucoma. 2014. PMID: 25275901 Free PMC article. Review.
-
A profile of transcriptomic changes in the rd10 mouse model of retinitis pigmentosa.Mol Vis. 2014 Nov 14;20:1612-28. eCollection 2014. Mol Vis. 2014. PMID: 25489233 Free PMC article.
-
An insight on established retinal injury mechanisms and prevalent retinal stem cell activation pathways in vertebrate models.Animal Model Exp Med. 2021 Jul 9;4(3):189-203. doi: 10.1002/ame2.12177. eCollection 2021 Sep. Animal Model Exp Med. 2021. PMID: 34557646 Free PMC article. Review.
-
Superior mechanical recovery in male and female MRL/MpJ tendons is associated with a unique genetic profile.J Orthop Res. 2021 Jun;39(6):1344-1354. doi: 10.1002/jor.24705. Epub 2020 Jun 8. J Orthop Res. 2021. PMID: 32352601 Free PMC article.
References
-
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. - PubMed
-
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. - PubMed
-
- Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. - PubMed
-
- Moon LD, Asher RA, Fawcett JW. Limited growth of severed CNS axons after treatment of adult rat brain with hyaluronidase. J Neurosci Res. 2003;71:23–37. - PubMed
-
- Chaitin MH, Wortham HS, Brun-Zinkernagel AM. Immunocyto-chemical localization of CD44 in the mouse retina. Exp Eye Res. 1994;58:359–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

