Probing the relative importance of molecular oscillations in the circadian clock
- PMID: 18385110
- PMCID: PMC2278066
- DOI: 10.1534/genetics.107.088658
Probing the relative importance of molecular oscillations in the circadian clock
Abstract
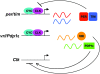
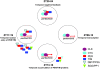
Circadian ( approximately 24 hr) rhythms of behavior and physiology are driven by molecular clocks that are endogenous to most organisms. The mechanisms underlying these clocks are remarkably conserved across evolution and typically consist of auto-regulatory loops in which specific proteins (clock proteins) rhythmically repress expression of their own genes. Such regulation maintains 24-hr cycles of RNA and protein expression. Despite the conservation of these mechanisms, however, questions are now being raised about the relevance of different molecular oscillations. Indeed, several studies have demonstrated that oscillations of some critical clock genes can be eliminated without loss of basic clock function. Here, we describe the multiple levels at which clock gene/protein expression and function can be rhythmically regulated-transcription, protein expression, post-translational modification, and localization-and speculate as to which aspect of this regulation is most critical. While the review is focused on Drosophila, we include some discussion of mammalian clocks to indicate the extent to which the questions concerning clock mechanisms are similar, regardless of the organism under study.
Figures
References
-
- Akten, B., E. Jauch, G. K. Genova, E. Y. Kim, I. Edery et al., 2003. A role for CK2 in the Drosophila circadian oscillator. Nat. Neurosci. 6 251–257. - PubMed
-
- Allada, R., N. E. White, W. V. So, J. C. Hall and M. Rosbash, 1998. A mutant Drosophila homolog of mammalian clock disrupts circadian rhythms and transcription of period and timeless. Cell 93 791–804. - PubMed
-
- Ashmore, L. J., and A. Sehgal, 2003. A fly's eye view of circadian entrainment. J. Biol. Rhythms 18 206–216. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

