Demyelinating and nondemyelinating strains of mouse hepatitis virus differ in their neural cell tropism
- PMID: 18385249
- PMCID: PMC2395180
- DOI: 10.1128/JVI.01488-07
Demyelinating and nondemyelinating strains of mouse hepatitis virus differ in their neural cell tropism
Abstract
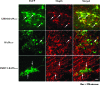
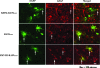
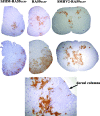
Some strains of mouse hepatitis virus (MHV) can induce chronic inflammatory demyelination in mice that mimics certain pathological features of multiple sclerosis. We have examined neural cell tropism of demyelinating and nondemyelinating strains of MHV in order to determine whether central nervous system (CNS) cell tropism plays a role in demyelination. Previous studies demonstrated that recombinant MHV strains, isogenic other than for the spike gene, differ in the extent of neurovirulence and the ability to induce demyelination. Here we demonstrate that these strains also differ in their abilities to infect a particular cell type(s) in the brain. Furthermore, there is a correlation between the differential localization of viral antigen in spinal cord gray matter and that in white matter during acute infection and the ability to induce demyelination later on. Viral antigen from demyelinating strains is detected initially in both gray and white matter, with subsequent localization to white matter of the spinal cord, whereas viral antigen localization of nondemyelinating strains is restricted mainly to gray matter. This observation suggests that the localization of viral antigen to white matter during the acute stage of infection is essential for the induction of chronic demyelination. Overall, these observations suggest that isogenic demyelinating and nondemyelinating strains of MHV, differing in the spike protein expressed, infect neurons and glial cells in different proportions and that differential tropism to a particular CNS cell type may play a significant role in mediating the onset and mechanisms of demyelination.
Figures

Similar articles
-
Macrophage-mediated optic neuritis induced by retrograde axonal transport of spike gene recombinant mouse hepatitis virus.J Neuropathol Exp Neurol. 2011 Jun;70(6):470-80. doi: 10.1097/NEN.0b013e31821da499. J Neuropathol Exp Neurol. 2011. PMID: 21572336 Free PMC article.
-
Mechanisms of primary axonal damage in a viral model of multiple sclerosis.J Neurosci. 2009 Aug 19;29(33):10272-80. doi: 10.1523/JNEUROSCI.1975-09.2009. J Neurosci. 2009. PMID: 19692601 Free PMC article.
-
Neurotropic Murine β-Coronavirus Infection Causes Differential Expression of Connexin 47 in Oligodendrocyte Subpopulations Associated with Demyelination.Mol Neurobiol. 2025 Mar;62(3):3428-3445. doi: 10.1007/s12035-024-04482-0. Epub 2024 Sep 18. Mol Neurobiol. 2025. PMID: 39292341 Free PMC article.
-
Pathogenesis of acute and chronic central nervous system infection with variants of mouse hepatitis virus, strain JHM.Immunol Res. 2007;39(1-3):160-72. doi: 10.1007/s12026-007-0079-y. Immunol Res. 2007. PMID: 17917063 Free PMC article. Review.
-
Pathogenesis of murine coronavirus in the central nervous system.J Neuroimmune Pharmacol. 2010 Sep;5(3):336-54. doi: 10.1007/s11481-010-9202-2. Epub 2010 Apr 6. J Neuroimmune Pharmacol. 2010. PMID: 20369302 Free PMC article. Review.
Cited by
-
Murine coronavirus delays expression of a subset of interferon-stimulated genes.J Virol. 2010 Jun;84(11):5656-69. doi: 10.1128/JVI.00211-10. Epub 2010 Mar 31. J Virol. 2010. PMID: 20357099 Free PMC article.
-
Virus infection, antiviral immunity, and autoimmunity.Immunol Rev. 2013 Sep;255(1):197-209. doi: 10.1111/imr.12091. Immunol Rev. 2013. PMID: 23947356 Free PMC article. Review.
-
Different mechanisms of inflammation induced in virus and autoimmune-mediated models of multiple sclerosis in C57BL6 mice.Biomed Res Int. 2013;2013:589048. doi: 10.1155/2013/589048. Epub 2013 Aug 28. Biomed Res Int. 2013. PMID: 24083230 Free PMC article.
-
Microglia play a major role in direct viral-induced demyelination.Clin Dev Immunol. 2013;2013:510396. doi: 10.1155/2013/510396. Epub 2013 Jun 20. Clin Dev Immunol. 2013. PMID: 23864878 Free PMC article.
-
Proline-Proline Dyad in the Fusion Peptide of the Murine β-Coronavirus Spike Protein's S2 Domain Modulates Its Neuroglial Tropism.Viruses. 2023 Jan 12;15(1):215. doi: 10.3390/v15010215. Viruses. 2023. PMID: 36680255 Free PMC article.
References
-
- Allen, I., and B. Brankin. 1993. Pathogenesis of multiple sclerosis—the immune diathesis and the role of viruses. J. Neuropathol. Exp. Neurol. 5295-105. - PubMed
-
- De Camilli, P., P. E. Miller, F. Navone, W. E. Theurkauf, and R. B. Vallee. 1984. Distribution of microtubule-associated protein 2 in the nervous system of the rat studied by immunofluorescence. Neuroscience 11817-846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

