Endoplasmic reticulum chaperones are involved in the morphogenesis of rotavirus infectious particles
- PMID: 18385250
- PMCID: PMC2395214
- DOI: 10.1128/JVI.02751-07
Endoplasmic reticulum chaperones are involved in the morphogenesis of rotavirus infectious particles
Abstract
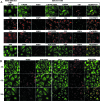
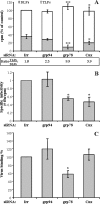
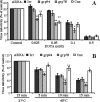
The final assembly of rotavirus particles takes place in the endoplasmic reticulum (ER). In this work, we evaluated by RNA interference the relevance to rotavirus assembly and infectivity of grp78, protein disulfide isomerase (PDI), grp94, calnexin, calreticulin, and ERp57, members of the two ER folding systems described herein. Silencing the expression of grp94 and Erp57 had no effect on rotavirus infectivity, while knocking down the expression of any of the other four chaperons caused a reduction in the yield of infectious virus of about 50%. In grp78-silenced cells, the maturation of the oligosaccharide chains of NSP4 was retarded. In cells with reduced levels of calnexin, the oxidative folding of VP7 was impaired and the trimming of NSP4 was accelerated, and in calreticulin-silenced cells, the formation of disulfide bonds of VP7 was also accelerated. The knockdown of PDI impaired the formation and/or rearrangement of the VP7 disulfide bonds. All these conditions also affected the correct assembly of virus particles, since compared with virions from control cells, they showed an altered susceptibility to EGTA and heat treatments, a decreased specific infectivity, and a diminished reactivity to VP7 with monoclonal antibody M60, which recognizes only this protein when its disulfide bonds have been correctly formed. In the case of grp78-silenced cells, the virus produced bound less efficiently to MA104 cells than virus obtained from control cells. All these results suggest that these chaperones are involved in the quality control of rotavirus morphogenesis. The complexity of the steps of rotavirus assembly that occur in the ER provide a useful model for studying the organization and operation of the complex network of chaperones involved in maintaining the quality control of this organelle.
Figures

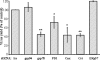


Similar articles
-
Silencing the morphogenesis of rotavirus.J Virol. 2005 Jan;79(1):184-92. doi: 10.1128/JVI.79.1.184-192.2005. J Virol. 2005. PMID: 15596814 Free PMC article.
-
COPII Vesicle Transport Is Required for Rotavirus NSP4 Interaction with the Autophagy Protein LC3 II and Trafficking to Viroplasms.J Virol. 2019 Dec 12;94(1):e01341-19. doi: 10.1128/JVI.01341-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597778 Free PMC article.
-
Calnexin, calreticulin, and ERp57 cooperate in disulfide bond formation in human CD1d heavy chain.J Biol Chem. 2002 Nov 22;277(47):44838-44. doi: 10.1074/jbc.M207831200. Epub 2002 Sep 17. J Biol Chem. 2002. PMID: 12239218
-
ERp57 and PDI: multifunctional protein disulfide isomerases with similar domain architectures but differing substrate-partner associations.Biochem Cell Biol. 2006 Dec;84(6):881-9. doi: 10.1139/o06-186. Biochem Cell Biol. 2006. PMID: 17215875 Review.
-
In vitro assays of the functions of calnexin and calreticulin, lectin chaperones of the endoplasmic reticulum.Methods Mol Biol. 2006;347:331-42. doi: 10.1385/1-59745-167-3:331. Methods Mol Biol. 2006. PMID: 17072021 Review.
Cited by
-
Protein disulfide isomerase is required for platelet-derived growth factor-induced vascular smooth muscle cell migration, Nox1 NADPH oxidase expression, and RhoGTPase activation.J Biol Chem. 2012 Aug 24;287(35):29290-300. doi: 10.1074/jbc.M112.394551. Epub 2012 Jul 6. J Biol Chem. 2012. PMID: 22773830 Free PMC article.
-
M1 of Murine Gamma-Herpesvirus 68 Induces Endoplasmic Reticulum Chaperone Production.Sci Rep. 2015 Nov 30;5:17228. doi: 10.1038/srep17228. Sci Rep. 2015. PMID: 26615759 Free PMC article.
-
The Guanine Nucleotide Exchange Factor GBF1 Participates in Rotavirus Replication.J Virol. 2019 Sep 12;93(19):e01062-19. doi: 10.1128/JVI.01062-19. Print 2019 Oct 1. J Virol. 2019. PMID: 31270230 Free PMC article.
-
The Role of Molecular Chaperones in Virus Infection and Implications for Understanding and Treating COVID-19.J Clin Med. 2020 Oct 30;9(11):3518. doi: 10.3390/jcm9113518. J Clin Med. 2020. PMID: 33143379 Free PMC article. Review.
-
GRP94 in ER quality control and stress responses.Semin Cell Dev Biol. 2010 Jul;21(5):479-85. doi: 10.1016/j.semcdb.2010.03.004. Epub 2010 Mar 16. Semin Cell Dev Biol. 2010. PMID: 20223290 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

