RPM-1, a Caenorhabditis elegans protein that functions in presynaptic differentiation, negatively regulates axon outgrowth by controlling SAX-3/robo and UNC-5/UNC5 activity
- PMID: 18385318
- PMCID: PMC6671101
- DOI: 10.1523/JNEUROSCI.5536-07.2008
RPM-1, a Caenorhabditis elegans protein that functions in presynaptic differentiation, negatively regulates axon outgrowth by controlling SAX-3/robo and UNC-5/UNC5 activity
Abstract
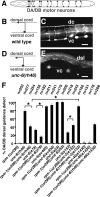
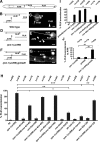
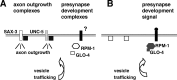
Changes in axon outgrowth patterns are often associated with synaptogenesis. Members of the conserved Pam/Highwire/RPM-1 protein family have essential functions in presynaptic differentiation. Here, we show that Caenorhabditis elegans RPM-1 negatively regulates axon outgrowth mediated by the guidance receptors SAX-3/robo and UNC-5/UNC5. Loss-of-function rpm-1 mutations cause a failure to terminate axon outgrowth, resulting in an overextension of the longitudinal PLM axon. We observe that PLM overextension in rpm-1 mutants is suppressed by sax-3 and unc-5 loss-of-function mutations. PLM axon overextension is also induced by SAX-3 overexpression, and the length of extension is enhanced by loss of rpm-1 function or suppressed by loss of unc-5 function. We also observe that loss of rpm-1 function in genetic backgrounds sensitized for guidance defects disrupts ventral AVM axon guidance in a SAX-3-dependent manner and enhances dorsal guidance of DA and DB motor axons in an UNC-5-dependent manner. Loss of rpm-1 function alters expression of the green fluorescent protein (GFP)-tagged proteins, SAX-3::GFP and UNC-5::GFP. RPM-1 is known to regulate axon termination through two parallel genetic pathways; one involves the Rab GEF (guanine nucleotide exchange factor) GLO-4, which regulates vesicular trafficking, and another that involves the F-box protein FSN-1, which mediates RPM-1 ubiquitin ligase activity. We show that glo-4 but not fsn-1 mutations affect axon guidance in a manner similar to loss of rpm-1 function. Together, the results suggest that RPM-1 regulates axon outgrowth affecting axon guidance and termination by controlling the trafficking of the UNC-5 and SAX-3 receptors to cell membranes.
Figures




Similar articles
-
C. elegans RPM-1 regulates axon termination and synaptogenesis through the Rab GEF GLO-4 and the Rab GTPase GLO-1.Neuron. 2007 Aug 16;55(4):587-601. doi: 10.1016/j.neuron.2007.07.009. Neuron. 2007. PMID: 17698012
-
Shared receptors in axon guidance: SAX-3/Robo signals via UNC-34/Enabled and a Netrin-independent UNC-40/DCC function.Nat Neurosci. 2002 Nov;5(11):1147-54. doi: 10.1038/nn956. Nat Neurosci. 2002. PMID: 12379860
-
SYD-1C, UNC-40 (DCC) and SAX-3 (Robo) function interdependently to promote axon guidance by regulating the MIG-2 GTPase.PLoS Genet. 2015 Apr 15;11(4):e1005185. doi: 10.1371/journal.pgen.1005185. eCollection 2015 Apr. PLoS Genet. 2015. PMID: 25876065 Free PMC article.
-
Netrin, Slit and Wnt receptors allow axons to choose the axis of migration.Dev Biol. 2008 Nov 15;323(2):143-51. doi: 10.1016/j.ydbio.2008.08.027. Epub 2008 Sep 5. Dev Biol. 2008. PMID: 18801355 Review.
-
Localization mechanisms of the axon guidance molecule UNC-6/Netrin and its receptors, UNC-5 and UNC-40, in Caenorhabditis elegans.Dev Growth Differ. 2012 Apr;54(3):390-7. doi: 10.1111/j.1440-169X.2012.01349.x. Dev Growth Differ. 2012. PMID: 22524608 Review.
Cited by
-
RPM-1 and DLK-1 regulate pioneer axon outgrowth by controlling Wnt signaling.Development. 2018 Sep 21;145(18):dev164897. doi: 10.1242/dev.164897. Development. 2018. PMID: 30093552 Free PMC article.
-
Axon Termination, Pruning, and Synaptogenesis in the Giant Fiber System of Drosophila melanogaster Is Promoted by Highwire.Genetics. 2017 Mar;205(3):1229-1245. doi: 10.1534/genetics.116.197343. Epub 2017 Jan 18. Genetics. 2017. PMID: 28100586 Free PMC article.
-
Developmental Function of the PHR Protein RPM-1 Is Required for Learning in Caenorhabditis elegans.G3 (Bethesda). 2015 Oct 13;5(12):2745-57. doi: 10.1534/g3.115.021410. G3 (Bethesda). 2015. PMID: 26464359 Free PMC article.
-
The role of ubiquitylation in nerve cell development.Nat Rev Neurosci. 2011 May;12(5):251-68. doi: 10.1038/nrn3009. Nat Rev Neurosci. 2011. PMID: 21505515 Review.
-
CLEC-38, a transmembrane protein with C-type lectin-like domains, negatively regulates UNC-40-mediated axon outgrowth and promotes presynaptic development in Caenorhabditis elegans.J Neurosci. 2008 Apr 23;28(17):4541-50. doi: 10.1523/JNEUROSCI.5542-07.2008. J Neurosci. 2008. PMID: 18434533 Free PMC article.
References
-
- Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, Culotti JG. UNC-40, a C. elegans homolog of DCC (deleted in colorectal cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. - PubMed
-
- Chang C, Adler CE, Krause M, Clark SG, Gertler FB, Tessier-Lavigne M, Bargmann CI. MIG-10/Lamellipodin and AGE-1/PI3K promote axon guidance and outgrowth in response to slit and netrin. Curr Biol. 2006;16:854–862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
