Interneuronal activity in reflex pathways from group II muscle afferents is monitored by dorsal spinocerebellar tract neurons in the cat
- PMID: 18385320
- PMCID: PMC6671081
- DOI: 10.1523/JNEUROSCI.0466-08.2008
Interneuronal activity in reflex pathways from group II muscle afferents is monitored by dorsal spinocerebellar tract neurons in the cat
Abstract
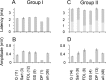
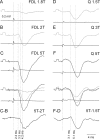
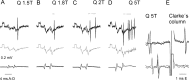
The main aim of the study was to investigate whether group II muscle afferents contribute to the inhibition of dorsal spinocerebellar tract (DSCT) neurons and thereby modulate information provided by these neurons in the cat. In intracellular recordings, we found disynaptic IPSPs from group II afferents in the majority of DSCT neurons, most often in parallel with IPSPs evoked from group I afferents. In an attempt to identify interneurons that mediate these IPSPs, the second aim of the study, laminas IV-VII in midlumbar segments were searched for interneurons antidromically activated by stimuli applied within Clarke's column. Such interneurons were found in regions in which focal field potentials were evoked by group I and II afferents, or ventral to them, and most were coexcited by these afferents. The input to these interneurons and their location indicate that they belonged to the previously identified population of premotor interneurons in disynaptic pathways between group I and II afferents and hindlimb motoneurons. The study leads thus to the conclusion that inhibitory actions of group II afferents on DSCT neurons are collateral to actions on motoneurons and that DSCT neurons monitor inhibitory actions of group II afferents on motoneurons as closely as they monitor actions of group I afferents. The results also indicate that interneurons mediating disynaptic reflex actions from tendon organ (group Ib) afferents and those mediating disynaptic actions from secondary muscle spindle (group II) afferents to motoneurons may be parts and parcel of the same interneuronal population rather than constitute distinct interneuronal populations.
Figures




Similar articles
-
Collateral actions of premotor interneurons on ventral spinocerebellar tract neurons in the cat.J Neurophysiol. 2010 Oct;104(4):1872-83. doi: 10.1152/jn.00408.2010. Epub 2010 Aug 11. J Neurophysiol. 2010. PMID: 20702737 Free PMC article.
-
Do premotor interneurons act in parallel on spinal motoneurons and on dorsal horn spinocerebellar and spinocervical tract neurons in the cat?J Neurophysiol. 2011 Apr;105(4):1581-93. doi: 10.1152/jn.00712.2010. Epub 2011 Jan 27. J Neurophysiol. 2011. PMID: 21273308 Free PMC article.
-
Same spinal interneurons mediate reflex actions of group Ib and group II afferents and crossed reticulospinal actions.J Neurophysiol. 2006 Jun;95(6):3911-22. doi: 10.1152/jn.01262.2005. Epub 2006 Mar 22. J Neurophysiol. 2006. PMID: 16554505 Free PMC article.
-
Interactions between spinal interneurons and ventral spinocerebellar tract neurons.J Physiol. 2013 Nov 15;591(22):5445-51. doi: 10.1113/jphysiol.2012.248740. Epub 2013 Jan 21. J Physiol. 2013. PMID: 23339177 Free PMC article. Review.
-
Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update.Eur J Neurosci. 2010 Sep;32(6):881-93. doi: 10.1111/j.1460-9568.2010.07354.x. Epub 2010 Aug 16. Eur J Neurosci. 2010. PMID: 20722720 Free PMC article. Review.
Cited by
-
Activity-dependent plasticity improves M1 motor representation and corticospinal tract connectivity.J Neurophysiol. 2009 Mar;101(3):1283-93. doi: 10.1152/jn.91026.2008. Epub 2008 Dec 17. J Neurophysiol. 2009. PMID: 19091920 Free PMC article.
-
A trans-spinal loop between neurones in the reticular formation and in the cerebellum.J Physiol. 2011 Feb 1;589(Pt 3):653-65. doi: 10.1113/jphysiol.2010.201178. Epub 2010 Dec 13. J Physiol. 2011. PMID: 21149461 Free PMC article.
-
Processing of multi-dimensional sensorimotor information in the spinal and cerebellar neuronal circuitry: a new hypothesis.PLoS Comput Biol. 2013;9(3):e1002979. doi: 10.1371/journal.pcbi.1002979. Epub 2013 Mar 14. PLoS Comput Biol. 2013. PMID: 23516353 Free PMC article.
-
Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit.Cell. 2009 Oct 2;139(1):161-74. doi: 10.1016/j.cell.2009.08.027. Cell. 2009. PMID: 19804761 Free PMC article.
-
Collateral actions of premotor interneurons on ventral spinocerebellar tract neurons in the cat.J Neurophysiol. 2010 Oct;104(4):1872-83. doi: 10.1152/jn.00408.2010. Epub 2010 Aug 11. J Neurophysiol. 2010. PMID: 20702737 Free PMC article.
References
-
- Bloedel JR, Courville J. Handbook of physiology. Bethesda, MD: American Physiological Society; 1981. Cerebellar afferent systems; pp. 735–829. The nervous system. Motor Control.
-
- Bosco G, Poppele RE. Proprioception from a spinocerebellar perspective. Physiol Rev. 2001;81:539–568. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
