Cyclin-dependent kinase 5 phosphorylation of human septin SEPT5 (hCDCrel-1) modulates exocytosis
- PMID: 18385322
- PMCID: PMC6671095
- DOI: 10.1523/JNEUROSCI.0453-08.2008
Cyclin-dependent kinase 5 phosphorylation of human septin SEPT5 (hCDCrel-1) modulates exocytosis
Abstract
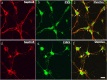
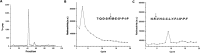
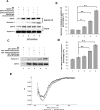
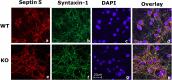
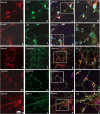
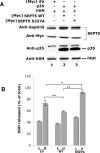
Cyclin-dependent kinase 5 (Cdk5) is predominantly expressed in the nervous system, where it is involved in neuronal migration, synaptic transmission, and survival. The role of Cdk5 in synaptic transmission is mediated by regulating the cellular functions of presynaptic proteins such as synapsin, Munc18, and dynamin 1. Its multifunctional role at the synapse is complex and probably involves other novel substrates. To explore this possibility, we used a yeast two-hybrid screen of a human cDNA library with p35 as bait and isolated human septin 5 (SEPT5), known also as hCDCrel-1, as an interacting clone. Here we report that p35 associates with SEPT5 in GST (glutathione S-transferase)-pull-down and coimmunoprecipitation assays. We confirmed that Cdk5/p35 phosphorylates SEPT5 in vitro and in vivo and identified S327 of SEPT5 as a major phosphorylation site. A serine (S)-to-alanine (A) 327 mutant of SEPT5 bound syntaxin more efficiently than SEPT5 wild type. Additionally, coimmunoprecipitation from synaptic vesicle fractions and Cdk5 wild-type and knock-out lysates showed that phosphorylation of septin 5 by Cdk5/p35 decreases its binding to syntaxin-1. Moreover, mutant nonphosphorylated SEPT5 potentiated regulated exocytosis more than the wild type when each was expressed in PC12 cells. These data suggest that Cdk5 phosphorylation of human septin SEPT5 at S327 plays a role in modulating exocytotic secretion.
Figures





References
-
- Amin ND, Albers W, Pant HC. Cyclin-dependent kinase 5 (cdk5) activation requires interaction with three domains of p35. J Neurosci Res. 2002;67:354–362. - PubMed
-
- Ballif BA, Villen J, Beausoleil SA, Schwartz D, Gygi SP. Phosphoproteomic analysis of the developing mouse brain. Mol Cell Proteomics. 2004;3:1093–1101. - PubMed
-
- Beites CL, Xie H, Bowser R, Trimble WS. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat Neurosci. 1999;2:434–439. - PubMed
-
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
