Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output
- PMID: 18385329
- PMCID: PMC6671086
- DOI: 10.1523/JNEUROSCI.4280-07.2008
Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output
Abstract
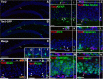
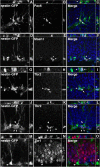
Neurogenesis in the adult hippocampus is a highly regulated process that originates from multipotent progenitors in the subgranular zone (SGZ). Currently, little is known about molecular mechanisms that regulate proliferation and differentiation in the SGZ. To study the role of transcription factors (TFs), we focused on Tbr2 (T-box brain gene 2), which has been implicated previously in developmental glutamatergic neurogenesis. In adult mouse hippocampus, Tbr2 protein and Tbr2-GFP (green fluorescent protein) transgene expression were specifically localized to intermediate-stage progenitor cells (IPCs), a type of transit amplifying cells. The Tbr2+ IPCs were highly responsive to neurogenic stimuli, more than doubling after voluntary wheel running. Notably, the Tbr2+ IPCs formed cellular clusters, the average size of which (Tbr2+ cells per cluster) likewise more than doubled in runners. Conversely, Tbr2+ IPCs were selectively depleted by antimitotic drugs, known to suppress neurogenesis. After cessation of antimitotic treatment, recovery of neurogenesis was paralleled by recovery of Tbr2+ IPCs, including a transient rebound above baseline numbers. Finally, Tbr2 was examined in the context of additional TFs that, together, define a TF cascade in embryonic neocortical neurogenesis (Pax6 --> Ngn2 --> Tbr2 --> NeuroD --> Tbr1). Remarkably, the same TF cascade was found to be linked to stages of neuronal lineage progression in adult SGZ. These results suggest that Tbr2+ IPCs play a major role in the regulation of adult hippocampal neurogenesis, and that a similar transcriptional program controls neurogenesis in adult SGZ as in embryonic cerebral cortex.
Figures





Similar articles
-
Tbr2 expression in Cajal-Retzius cells and intermediate neuronal progenitors is required for morphogenesis of the dentate gyrus.J Neurosci. 2013 Feb 27;33(9):4165-80. doi: 10.1523/JNEUROSCI.4185-12.2013. J Neurosci. 2013. PMID: 23447624 Free PMC article.
-
Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis.Eur J Neurosci. 2009 Jan;29(2):232-43. doi: 10.1111/j.1460-9568.2008.06595.x. Eur J Neurosci. 2009. PMID: 19200230
-
Tbr2 is essential for hippocampal lineage progression from neural stem cells to intermediate progenitors and neurons.J Neurosci. 2012 May 2;32(18):6275-87. doi: 10.1523/JNEUROSCI.0532-12.2012. J Neurosci. 2012. PMID: 22553033 Free PMC article.
-
Control of Neuronal Development by T-Box Genes in the Brain.Curr Top Dev Biol. 2017;122:279-312. doi: 10.1016/bs.ctdb.2016.08.001. Epub 2016 Sep 1. Curr Top Dev Biol. 2017. PMID: 28057268 Review.
-
Intermediate progenitors and Tbr2 in cortical development.J Anat. 2019 Sep;235(3):616-625. doi: 10.1111/joa.12939. Epub 2019 Jan 24. J Anat. 2019. PMID: 30677129 Free PMC article. Review.
Cited by
-
A dual function of Bcl11b/Ctip2 in hippocampal neurogenesis.EMBO J. 2012 Jun 29;31(13):2922-36. doi: 10.1038/emboj.2012.142. Epub 2012 May 15. EMBO J. 2012. PMID: 22588081 Free PMC article.
-
The Origin, Development and Molecular Diversity of Rodent Olfactory Bulb Glutamatergic Neurons Distinguished by Expression of Transcription Factor NeuroD1.PLoS One. 2015 Jun 1;10(6):e0128035. doi: 10.1371/journal.pone.0128035. eCollection 2015. PLoS One. 2015. PMID: 26030886 Free PMC article.
-
Regulation of organogenesis and stem cell properties by T-box transcription factors.Cell Mol Life Sci. 2013 Oct;70(20):3929-45. doi: 10.1007/s00018-013-1305-5. Epub 2013 Mar 12. Cell Mol Life Sci. 2013. PMID: 23479132 Free PMC article. Review.
-
Early life stress delays hippocampal development and diminishes the adult stem cell pool in mice.Sci Rep. 2019 Mar 11;9(1):4120. doi: 10.1038/s41598-019-40868-0. Sci Rep. 2019. PMID: 30858462 Free PMC article.
-
AAV ablates neurogenesis in the adult murine hippocampus.Elife. 2021 Jul 14;10:e59291. doi: 10.7554/eLife.59291. Elife. 2021. PMID: 34259630 Free PMC article.
References
-
- Arlotta P, Molyneaux B, Chen J, Inoue J, Kominami R, Macklis J. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. - PubMed
-
- Britz O, Mattar P, Nguyen L, Langevin L, Zimmer C, Alam S, Guillemot F, Schuurmans C. A role for proneural genes in the maturation of cortical progenitor cells. Cereb Cortex. 2006;16:i138–i151. - PubMed
-
- Emsley J, Mitchell B, Kempermann G, Macklis J. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
