Lessons for successful study enrollment from the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study
- PMID: 18385390
- PMCID: PMC2440269
- DOI: 10.2215/CJN.05621207
Lessons for successful study enrollment from the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study
Abstract
Background and objectives: Design elements of clinical trials can introduce recruitment bias and reduce study efficiency. Trials involving the critically ill may be particularly prone to design-related inefficiencies.
Design, setting, participants, & measurements: Enrollment into the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study was systematically monitored. Reasons for nonenrollment into this study comparing strategies of renal replacement therapy in critically ill patients with acute kidney injury were categorized as modifiable or nonmodifiable.
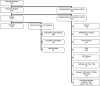
Results: 4339 patients were screened; 2744 fulfilled inclusion criteria. Of these, 1034 were ineligible by exclusion criteria. Of the remaining 1710 patients, 1124 (65.7%) enrolled. Impediments to informed consent excluded 21.4% of potentially eligible patients. Delayed identification of potential patients, physician refusal, and involvement in competing trials accounted for 4.4, 2.7, and 2.3% of exclusions. Comfort measures only status, chronic illness, chronic kidney disease, and obesity excluded 11.8, 7.8, 7.6, and 5.9% of potential patients. Modification of an enrollment window reduced the loss of patients from 6.6 to 2.3%.
Conclusions: The Acute Renal Failure Trial Network Study's enrollment efficiency compared favorably with previous intensive care unit intervention trials and supports the representativeness of its enrolled population. Impediments to informed consent highlight the need for nontraditional acquisition methods. Restrictive enrollment windows may hamper recruitment but can be effectively modified. The low rate of physician refusal acknowledges clinical equipoise in the study design. Underlying comorbidities are important design considerations for future trials that involve the critically ill with acute kidney injury.
Figures
References
-
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, Gotzsche PC, Lang T: The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Ann Intern Med 134: 663–694, 2001 - PubMed
-
- Moher D, Schulz KF, Altman DG, Consort G: The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med 134: 657–662, 2001 - PubMed
-
- Shapiro SH, Weijer C, Freedman B: Reporting the study populations of clinical trials: Clear transmission or static on the line? J Clin Epidemiol 53: 973–979, 2000 - PubMed
-
- Gandhi M, Ameli N, Bacchetti P, Sharp GB, French AL, Young M, Gange SJ, Anastos K, Holman S, Levine A, Greenblatt RM: Eligibility criteria for HIV clinical trials and generalizability of results: The gap between published reports and study protocols. AIDS 19: 1885–1896, 2005 - PubMed
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkim I, Pitkin R, Rennie D, Schulz K, Simel D, Stroup D: Improving the quality of reporting of randomized controlled trials: The CONSORT statement. JAMA 276: 637–639, 1996 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical