LRRC50, a conserved ciliary protein implicated in polycystic kidney disease
- PMID: 18385425
- PMCID: PMC2396934
- DOI: 10.1681/ASN.2007080917
LRRC50, a conserved ciliary protein implicated in polycystic kidney disease
Abstract
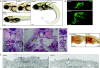
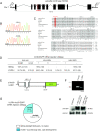
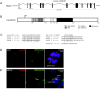
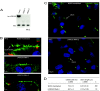
Cilia perform essential motile and sensory functions central to many developmental and physiological processes. Disruption of their structure or function can have profound phenotypic consequences, and has been linked to left-right patterning and polycystic kidney disease. In a forward genetic screen for mutations affecting ciliary motility, we isolated zebrafish mutant hu255H. The mutation was found to disrupt an ortholog of the uncharacterized highly conserved human SDS22-like leucine-rich repeat(LRR)-containing protein LRRC50 (16q24.1) and Chlamydomonas Oda7p. Zebrafish lrrc50 is specifically expressed in all ciliated tissues. lrrc50(hu255H) mutants develop pronephric cysts with an increased proliferative index, severely reduced brush border, and disorganized pronephric cilia manifesting impaired localized fluid flow consistent with ciliary dysfunction. Electron microscopy analysis revealed ultrastructural irregularities of the dynein arms and misalignments of the outer-doublet microtubules on the ciliary axonemes, suggesting instability of the ciliary architecture in lrrc50(hu255H) mutants. TheSDS22-like leucine-rich repeats present in Lrrc50 are necessary for proper protein function, since injection of a deletion construct of the first LRR did not rescue the zebrafish mutant phenotype. Subcellular distribution of human LRRC50-EGFP in MDCK and HEK293T cells is diffusely cytoplasmic and concentrated at the mitotic spindle poles and cilium. LRRC50 RNAi knock-down in human proximal tubule HK-2 cells thoroughly recapitulated the zebrafish brush border and cilia phenotype, suggesting conservation of LRRC50 function between both species. In summary, we present the first genetic vertebrate model for lrrc50 function and propose LRRC50 to be a novel candidate gene for human cystic kidney disease, involved in regulation of microtubule-based cilia and actin-based brush border microvilli.
Figures



References
-
- Ibanez-Tallon I, Heintz N, Omran H: To beat or not to beat: Roles of cilia in development and disease. Hum Mol Genet 12: R27–R35, 2003 - PubMed
-
- Brokaw CJ: Flagellar movement: A sliding filament model. Science 178: 455–462, 1972 - PubMed
-
- Dutcher SK: Flagellar assembly in two hundred and fifty easy-to-follow steps. Trends Genet 11: 398–404, 1995 - PubMed
-
- Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, Lewis RA, Green JS, Parfrey PS, Leroux MR, Davidson WS, Beales PL, Guay-Woodford LM, Yoder BK, Stormo GD, Katsanis N, Dutcher SK: Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell 117: 541–552, 2004 - PubMed
-
- Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N: A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131: 4085–4093, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

