Acute kidney injury leads to inflammation and functional changes in the brain
- PMID: 18385426
- PMCID: PMC2440297
- DOI: 10.1681/ASN.2007080901
Acute kidney injury leads to inflammation and functional changes in the brain
Abstract
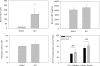
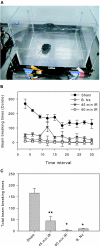
Although neurologic sequelae of acute kidney injury (AKI) are well described, the pathogenesis of acute uremic encephalopathy is poorly understood. This study examined the short-term effect of ischemic AKI on inflammatory and functional changes of the brain in mice by inducing bilateral renal ischemia for 60 min and studying the brains 24 h later. Compared with sham mice, mice with AKI had increased neuronal pyknosis and microgliosis in the brain. AKI also led to increased levels of the proinflammatory chemokines keratinocyte-derived chemoattractant and G-CSF in the cerebral cortex and hippocampus and increased expression of glial fibrillary acidic protein in astrocytes in the cortex and corpus callosum. In addition, extravasation of Evans blue dye into the brain suggested that the blood-brain barrier was disrupted in mice with AKI. Because liver failure also leads to encephalopathy, ischemic liver injury was induced in mice with normal renal function; neuronal pyknosis and glial fibrillary acidic protein expression were not increased, suggesting differential effects on the brain depending on the organ injured. For evaluation of the effects of AKI on brain function, locomotor activity was studied using an open field test. Mice subjected to renal ischemia or bilateral nephrectomy had moderate to severe declines in locomotor activity compared with sham-operated mice. These data demonstrate that severe ischemic AKI induces inflammation and functional changes in the brain. Targeting these pathways could reduce morbidity and mortality in critically ill patients with severe AKI.
Figures
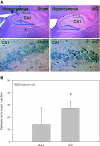

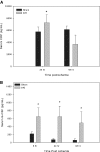
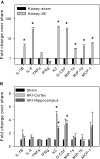
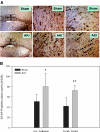

Comment in
-
Marconi revisited: from kidney to brain--two organ systems communicating at long distance.J Am Soc Nephrol. 2008 Jul;19(7):1253-5. doi: 10.1681/ASN.2008040404. Epub 2008 May 28. J Am Soc Nephrol. 2008. PMID: 18508961 No abstract available.
References
-
- Rabb H, Chamoun F, Hotchkiss J: Molecular mechanisms underlying combined kidney-lung dysfunction during acute renal failure. Contrib Nephrol 41–52, 2001 - PubMed
-
- Kelly KJ: Acute renal failure: much more than a kidney disease. Semin Nephrol 26: 105–113, 2006 - PubMed
-
- Star RA: Treatment of acute renal failure. Kidney Int 54: 1817–1831, 1998 - PubMed
-
- Brouns R, De Deyn PP: Neurological complications in renal failure: A review. Clin Neurol Neurosurg 107: 1–16, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

