Src inhibition ameliorates polycystic kidney disease
- PMID: 18385429
- PMCID: PMC2440293
- DOI: 10.1681/ASN.2007060665
Src inhibition ameliorates polycystic kidney disease
Abstract
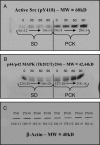
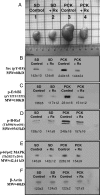
Despite identification of the genes responsible for autosomal dominant polycystic kidney disease (PKD) and autosomal recessive PKD (ARPKD), the precise functions of their cystoprotein products remain unknown. Recent data suggested that multimeric cystoprotein complexes initiate aberrant signaling cascades in PKD, and common components of these signaling pathways may be therapeutic targets. This study identified c-Src (pp60(c-Src)) as one such common signaling intermediate and sought to determine whether Src activity plays a role in cyst formation. With the use of the nonorthologous BPK murine model and the orthologous PCK rat model of ARPKD, greater Src activity was found to correlate with disease progression. Inhibition of Src activity with the pharmacologic inhibitor SKI-606 resulted in amelioration of renal cyst formation and biliary ductal abnormalities in both models. Furthermore, the effects of Src inhibition in PCK kidneys suggest that the ErbB2 and B-Raf/MEK/ERK pathways are involved in Src-mediated signaling in ARPKD and that this occurs without reducing elevated cAMP. These data suggest that Src inhibition may provide therapeutic benefit in PKD.
Figures







Similar articles
-
20-HETE activates the Raf/MEK/ERK pathway in renal epithelial cells through an EGFR- and c-Src-dependent mechanism.Am J Physiol Renal Physiol. 2009 Sep;297(3):F662-70. doi: 10.1152/ajprenal.00146.2009. Epub 2009 Jul 1. Am J Physiol Renal Physiol. 2009. PMID: 19570883 Free PMC article.
-
c-Src inactivation reduces renal epithelial cell-matrix adhesion, proliferation, and cyst formation.Am J Physiol Cell Physiol. 2011 Aug;301(2):C522-9. doi: 10.1152/ajpcell.00163.2010. Epub 2011 Apr 20. Am J Physiol Cell Physiol. 2011. PMID: 21508333 Free PMC article.
-
Chronic blockade of 20-HETE synthesis reduces polycystic kidney disease in an orthologous rat model of ARPKD.Am J Physiol Renal Physiol. 2009 Mar;296(3):F575-82. doi: 10.1152/ajprenal.90705.2008. Epub 2009 Jan 7. Am J Physiol Renal Physiol. 2009. PMID: 19129252 Free PMC article.
-
Proliferative signaling by ERBB proteins and RAF/MEK/ERK effectors in polycystic kidney disease.Cell Signal. 2020 Mar;67:109497. doi: 10.1016/j.cellsig.2019.109497. Epub 2019 Dec 9. Cell Signal. 2020. PMID: 31830556 Free PMC article. Review.
-
Molecular and cellular pathophysiology of autosomal recessive polycystic kidney disease (ARPKD).Cell Tissue Res. 2006 Dec;326(3):671-85. doi: 10.1007/s00441-006-0226-0. Epub 2006 Jun 10. Cell Tissue Res. 2006. PMID: 16767405 Review.
Cited by
-
CDK inhibitors R-roscovitine and S-CR8 effectively block renal and hepatic cystogenesis in an orthologous model of ADPKD.Cell Cycle. 2012 Nov 1;11(21):4040-6. doi: 10.4161/cc.22375. Epub 2012 Oct 3. Cell Cycle. 2012. PMID: 23032260 Free PMC article.
-
Discoidin Domain Receptor 1 (DDR1) tyrosine kinase is upregulated in PKD kidneys but does not play a role in the pathogenesis of polycystic kidney disease.PLoS One. 2019 Jul 1;14(7):e0211670. doi: 10.1371/journal.pone.0211670. eCollection 2019. PLoS One. 2019. PMID: 31260458 Free PMC article.
-
Pathophysiology of childhood polycystic kidney diseases: new insights into disease-specific therapy.Pediatr Res. 2014 Jan;75(1-2):148-57. doi: 10.1038/pr.2013.191. Epub 2013 Oct 31. Pediatr Res. 2014. PMID: 24336431 Free PMC article. Review.
-
Evaluation of metalloprotease inhibitors on hypertension and diabetic nephropathy.Am J Physiol Renal Physiol. 2011 Apr;300(4):F983-98. doi: 10.1152/ajprenal.00262.2010. Epub 2011 Jan 12. Am J Physiol Renal Physiol. 2011. PMID: 21228113 Free PMC article.
-
Inhibiting heat shock protein 90 (HSP90) limits the formation of liver cysts induced by conditional deletion of Pkd1 in mice.PLoS One. 2014 Dec 4;9(12):e114403. doi: 10.1371/journal.pone.0114403. eCollection 2014. PLoS One. 2014. PMID: 25474361 Free PMC article.
References
-
- Ong AC, Harris PC: Molecular pathogenesis of ADPKD: The polycystin complex gets complex. Kidney Int 67: 1234–1247, 2005 - PubMed
-
- Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC: The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 30: 259–269, 2002 - PubMed
-
- Onuchic LF, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, Rudnik-Schoneborn S, Mrug M, Sweeney W, Avner ED, Zerres K, Guay-Woodford LM, Somlo S, Germino GG: PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet 70: 1305–1317, 2002 - PMC - PubMed
-
- Dell K, McDonald R, Watkins SL, Avner ED: Polycystic kidney disease. In: Pediatric Nephrology, 5th Ed., edited by Avner ED, Harmon WE, Niaudet P, Philadelphia, Lippincott Williams & Wilkins, 2004, pp 675–699
-
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB: Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12: R378–R380, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

