A supraphysiological nuclear export signal is required for parvovirus nuclear export
- PMID: 18385513
- PMCID: PMC2397317
- DOI: 10.1091/mbc.e08-01-0009
A supraphysiological nuclear export signal is required for parvovirus nuclear export
Abstract
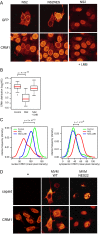
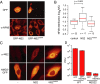
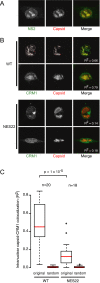
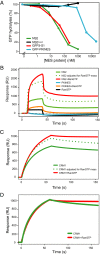
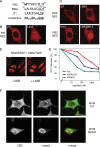
CRM1 exports proteins that carry a short leucine-rich peptide signal, the nuclear export signal (NES), from the nucleus. Regular NESs must have low affinity for CRM1 to function optimally. We previously generated artificial NESs with higher affinities for CRM1, termed supraphysiological NESs. Here we identify a supraphysiological NES in an endogenous protein, the NS2 protein of parvovirus Minute Virus of Mice (MVM). NS2 interacts with CRM1 without the requirement of RanGTP, whereas addition of RanGTP renders the complex highly stable. Mutation of a single hydrophobic residue that inactivates regular NESs lowers the affinity of the NS2 NES for CRM1 from supraphysiological to regular. Mutant MVM harboring this regular NES is compromised in viral nuclear export and productivity. In virus-infected mouse fibroblasts we observe colocalization of NS2, CRM1 and mature virions, which is dependent on the supraphysiological NS2 NES. We conclude that supraphysiological NESs exist in nature and that the supraphysiological NS2 NES has a critical role in active nuclear export of mature MVM particles before cell lysis.
Figures

References
-
- Bischoff F. R., Gorlich D. RanBP1 is crucial for the release of RanGTP from importin beta-related nuclear transport factors. FEBS Lett. 1997;419:249–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

