Activation of keratinocyte protein kinase C zeta in psoriasis plaques
- PMID: 18385757
- PMCID: PMC3120228
- DOI: 10.1038/jid.2008.81
Activation of keratinocyte protein kinase C zeta in psoriasis plaques
Abstract
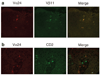
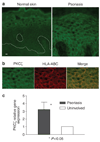
PKCzeta (protein kinase C-zeta), a member of protein kinase C family, plays an important role in cell proliferation, differentiation, and apoptosis. It acts as a downstream molecule for TNF-alpha (tumor necrosis factor) signal transduction and also regulates the expression of CD1d, an HLA-class I-like molecule. The interaction of CD1d with natural killer T (NKT) cells has been shown to be important in their Th1 cytokine production in psoriasis. In this study, we examined PKCzeta in psoriasis in order to define its role in the pathogenesis of the disease. We found that T-cell receptor (TCR) V alpha24+ V beta11+ NKT cells and CD1d molecules within psoriatic skin were increased. Moreover, there was an associated increase in PKCzeta mRNA and protein expression with membrane translocation in psoriasis lesions compared to uninvolved skin. Furthermore, cultured keratinocytes exhibited increased PKCzeta activity and membrane translocation upon stimulation by TNF-alpha, a cytokine known to play an important role in the pathogenesis of psoriasis. These results implied that PKCzeta is an important transduction molecule downstream of TNF-alpha signaling and is associated with increased expression of CD1d that may enhance CD1d-NKT cell interactions in psoriasis lesions. This makes PKCzeta a tempting target for possible pharmacological intervention in modifying the downstream effects of TNF-alpha in psoriasis.
Conflict of interest statement
The authors state no conflict of interest.
Figures


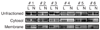


Similar articles
-
Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-gamma production by NK-T cells.J Immunol. 2000 Oct 1;165(7):4076-85. doi: 10.4049/jimmunol.165.7.4076. J Immunol. 2000. PMID: 11034419
-
Human invariant V alpha 24-J alpha Q TCR supports the development of CD1d-dependent NK1.1+ and NK1.1- T cells in transgenic mice.J Immunol. 2003 Mar 1;170(5):2390-8. doi: 10.4049/jimmunol.170.5.2390. J Immunol. 2003. PMID: 12594262
-
Activation of ribosomal protein S6 kinase in psoriatic lesions and cultured human keratinocytes by epidermal growth factor receptor ligands.J Invest Dermatol. 1997 Jan;108(1):98-102. doi: 10.1111/1523-1747.ep12285647. J Invest Dermatol. 1997. PMID: 8980296
-
Increased mRNA expression of manganese superoxide dismutase in psoriasis skin lesions and in cultured human keratinocytes exposed to IL-1 beta and TNF-alpha.Free Radic Biol Med. 1995 Feb;18(2):349-55. doi: 10.1016/0891-5849(94)e0124-2. Free Radic Biol Med. 1995. PMID: 7744320
-
The Role of Adaptor Proteins in the Biology of Natural Killer T (NKT) Cells.Front Immunol. 2019 Jun 25;10:1449. doi: 10.3389/fimmu.2019.01449. eCollection 2019. Front Immunol. 2019. PMID: 31293596 Free PMC article. Review.
Cited by
-
Therapeutic manipulation of natural killer (NK) T cells in autoimmunity: are we close to reality?Clin Exp Immunol. 2013 Jan;171(1):8-19. doi: 10.1111/j.1365-2249.2012.04625.x. Clin Exp Immunol. 2013. PMID: 23199318 Free PMC article. Review.
-
Dysregulated expression of inflammation-related genes in psoriatic dermis mesenchymal stem cells.Acta Biochim Biophys Sin (Shanghai). 2016 Jun;48(6):587-8. doi: 10.1093/abbs/gmw036. Epub 2016 May 4. Acta Biochim Biophys Sin (Shanghai). 2016. PMID: 27151294 Free PMC article. No abstract available.
-
Herpesvirus entry mediator regulates hypoxia-inducible factor-1α and erythropoiesis in mice.J Clin Invest. 2011 Dec;121(12):4810-9. doi: 10.1172/JCI57332. Epub 2011 Nov 14. J Clin Invest. 2011. PMID: 22080867 Free PMC article.
-
Th17/Tc17 infiltration and associated cytokine gene expression in elicitation phase of allergic contact dermatitis.Br J Dermatol. 2009 Dec;161(6):1301-6. doi: 10.1111/j.1365-2133.2009.09400.x. Epub 2009 Jul 7. Br J Dermatol. 2009. PMID: 19785613 Free PMC article.
-
Effects of moving cupping therapy for plaque psoriasis: study protocol for a randomized multicenter clinical trial.Trials. 2020 Feb 26;21(1):229. doi: 10.1186/s13063-020-4155-0. Trials. 2020. PMID: 32102679 Free PMC article.
References
-
- Bonish B, Jullien D, Dutronc Y, Huang BB, Modlin R, Spada FM, et al. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-gamma production by NK-T cells. J Immunol. 2000;165:4076–4085. - PubMed
-
- Bos JD. Psoriasis, innate immunity, and gene pools. J Am Acad Dermatol. 2007;56:468–471. - PubMed
-
- Cameron AL, Kirby B, Fei W, Griffiths CE. Natural killer and natural killer-T cells in psoriasis. Arch Dermatol Res. 2002;294:363–369. - PubMed
-
- Cohen EE, Lingen MW, Zhu B, Zhu H, Straza MW, Pierce C, et al. Protein kinase C zeta mediates epidermal growth factor-induced growth of head and neck tumor cells by regulating mitogen-activated protein kinase. Cancer Res. 2006;66:6296–6303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

