Promiscuous recombination of LoxP alleles during gametogenesis in cornea Cre driver mice
- PMID: 18385792
- PMCID: PMC2274927
Promiscuous recombination of LoxP alleles during gametogenesis in cornea Cre driver mice
Abstract
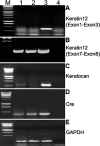
Purpose: To examine whether promiscuous Cre/LoxP recombination happens during gametogenesis in double transgenic mice carrying LoxP modified alleles and Cre transgene driven by tissue-specific promoter outside the gonads of adult mice.
Methods: Cre driver mice were crossbred with reporter mouse lines (e.g., ZEG and Rosa26R) to obtain Cre/ZEG and Cre/Rosa26R double transgenic mice. The frequency of promiscuous LoxP/Cre recombination was determined by the expression of second reporter genes in the offspring of double transgenic mice.
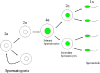
Results: The frequency of promiscuous LoxP/Cre recombination varied in different lines of Cre driver mice and in the sex of the same driver mice with higher penetrance in male than in female double transgenic mice. Polymerase chain reaction (PCR) and recombination analysis demonstrate that the recombination of floxed allele occurs during the transition from spermatogonia (diploid) to primary spermatocyte (tetraploid) in the testis. Thereby, target-floxed allele(s) may be ubiquitously ablated in experimental animals intended for tissue-specific gene deletion.
Conclusions: Gametogenesis-associated recombination should always be examined in tissue-specific gene ablation studies.
Figures





Similar articles
-
Non-parallel recombination limits Cre-LoxP-based reporters as precise indicators of conditional genetic manipulation.Genesis. 2013 Jun;51(6):436-42. doi: 10.1002/dvg.22384. Epub 2013 Mar 26. Genesis. 2013. PMID: 23441020 Free PMC article.
-
Generation and characterization of Ins1-cre-driver C57BL/6N for exclusive pancreatic beta cell-specific Cre-loxP recombination.Exp Anim. 2014;63(2):183-91. doi: 10.1538/expanim.63.183. Exp Anim. 2014. PMID: 24770644 Free PMC article.
-
Cre-mediated recombination efficiency and transgene expression patterns of three retinal bipolar cell-expressing Cre transgenic mouse lines.Mol Vis. 2013 Jun 12;19:1310-20. Print 2013. Mol Vis. 2013. PMID: 23805038 Free PMC article.
-
Pancreas-specific Cre driver lines and considerations for their prudent use.Cell Metab. 2013 Jul 2;18(1):9-20. doi: 10.1016/j.cmet.2013.06.011. Cell Metab. 2013. PMID: 23823474 Free PMC article. Review.
-
Perspective: The current state of Cre driver mouse lines in skeletal research: Challenges and opportunities.Bone. 2023 May;170:116719. doi: 10.1016/j.bone.2023.116719. Epub 2023 Mar 1. Bone. 2023. PMID: 36868507 Free PMC article. Review.
Cited by
-
Simultaneous deletion of floxed genes mediated by CaMKIIα-Cre in the brain and in male germ cells: application to conditional and conventional disruption of Goα.Exp Mol Med. 2014 May 2;46(5):e93. doi: 10.1038/emm.2014.14. Exp Mol Med. 2014. PMID: 24787734 Free PMC article.
-
Collagen V is a dominant regulator of collagen fibrillogenesis: dysfunctional regulation of structure and function in a corneal-stroma-specific Col5a1-null mouse model.J Cell Sci. 2011 Dec 1;124(Pt 23):4096-105. doi: 10.1242/jcs.091363. Epub 2011 Dec 8. J Cell Sci. 2011. PMID: 22159420 Free PMC article.
-
Generation and characterization of Aldh3-Cre transgenic mice as a tool for conditional gene deletion in postnatal cornea.Sci Rep. 2020 Jun 3;10(1):9083. doi: 10.1038/s41598-020-65878-1. Sci Rep. 2020. PMID: 32493941 Free PMC article.
-
Bone marrow mesenchymal stem cells can differentiate and assume corneal keratocyte phenotype.J Cell Mol Med. 2012 May;16(5):1114-24. doi: 10.1111/j.1582-4934.2011.01418.x. J Cell Mol Med. 2012. PMID: 21883890 Free PMC article.
-
Hemizygous Le-Cre transgenic mice have severe eye abnormalities on some genetic backgrounds in the absence of LoxP sites.PLoS One. 2014 Oct 1;9(10):e109193. doi: 10.1371/journal.pone.0109193. eCollection 2014. PLoS One. 2014. PMID: 25272013 Free PMC article.
References
-
- Porter A. Controlling your losses: conditional gene silencing in mammals. Trends Genet. 1998;14:73–9. - PubMed
-
- Tanifuji-Terai N, Terai K, Hayashi Y, Chikama T, Kao WW. Expression of Keratin 12 and Maturation of Corneal Epithelium during Development and Postnatal Growth. Invest Ophthalmol Vis Sci. 2006;47:545–51. - PubMed
-
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. - PubMed
-
- Liu CY, Shiraishi A, Kao CW, Converse RL, Funderburgh JL, Corpuz LM, Conrad GW, Kao WW. The cloning of mouse keratocan cDNA and genomic DNA and the characterization of its expression during eye development. J Biol Chem. 1998;273:22584–8. - PubMed
-
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
