Beta1-6 branching of cell surface glycoproteins may contribute to uveal melanoma progression by up-regulating cell motility
- PMID: 18385798
- PMCID: PMC2276181
Beta1-6 branching of cell surface glycoproteins may contribute to uveal melanoma progression by up-regulating cell motility
Abstract
Purpose: This study investigated the influence of integrin expression as well as the oligosaccharide structure of surface N-glycoproteins on cell behavior of two primary uveal (92-1 and Mel202) and two primary cutaneous (FM55P and IGR-39) melanoma cell lines.
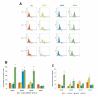
Methods: Cell adhesion to fibronectin and cell migration on fibronectin (wound healing) were selected as the studied cell behavior parameters. The percentage of cells positive for expression of selected integrins was estimated by flow cytometric analysis. The influence of beta1-6 branched complex-type N-oligosaccharides on wound healing on fibronectin was investigated. Cell surface beta1-6 branched N-oligosaccharides were measured by their specific binding to PHA-L followed by flow cytometry, and the fibronectin receptors bearing beta1-6 GlcNAc branched N-linked glycans were identified. In addition, the transcript of GnT-V (the enzyme that catalyzes the addition of N-acetylglucosamine to the core mannose of di- and tri-antennary N-glycans through a beta1-6 linkage) was analyzed by semiquantitative RT-PCR.
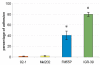
Results: Unlike the two examined cutaneous melanoma cell lines, neither of the uveal melanoma cells adhered to fibronectin. The adhesion efficiency of IGR-39 cells was twice that of FM55P cells. In contrast, uveal melanoma cells repaired scratch wounds on fibronectin-coated surfaces twice as fast as cutaneous melanoma cells did. The expression of alpha(3)beta(1), alpha(4)beta(1), alpha(5)beta(1), and alpha(v)beta(3) integrins, acting as fibronectin receptors, differed between the tested cell lines, and no distinct pattern distinguished uveal melanoma from cutaneous melanoma except for high expression of alpha(4)beta(1) integrin on both FM55P and IGR-39 cells. The results also demonstrated that the high levels of alpha(3)beta(1), alpha(4)beta(1), and alpha(5)beta(1) integrin expression on IGR-39 cells promoted their strong attachment to fibronectin-coated surfaces. In addition, 92-1, Mel202, and FM55P cells showed no or low adhesion to fibronectin, perhaps the result of low expression of fibronectin receptors excluding high expression of alpha(4)beta(1) integrin in FM55P cells. Cell migration was significantly decreased in three out of four PHA-L-treated cell lines, suggesting that beta1-6 branched complex type N-oligosaccharides are critical for 92-1, Mel202, and FM55P cell motility. Semiquantitative RT-PCR analysis showed that the tested cells did not differ in mRNA levels of beta1-6 -N-acetylglucosaminyltransferase V. However, FACS analysis showed that 92-1, Mel202 and IGR-39 cells expressed significantly higher amounts of beta1-6 branched N-oligosaccharides on the cell surface than FM55P cells did. All examined alpha(3), alpha(5), alpha(v), and beta(1) integrin subunits were shown to bear beta1-6 branched N-linked glycans.
Conclusions: The role of integrins and their N-glycosylation in the regulation of uveal melanoma growth and progression is largely unknown. These results reveal that cell surface complex-type N-glycans with GlcNAc beta1-6 branches are important factors determining the migration of primary uveal melanoma cells on fibronectin.
Figures



Similar articles
-
Studies on primary uveal and cutaneous melanoma cell interaction with vitronectin.Cell Biol Int. 2014 Aug;38(8):942-52. doi: 10.1002/cbin.10280. Epub 2014 Apr 28. Cell Biol Int. 2014. PMID: 24687613
-
Diverse expression of N-acetylglucosaminyltransferase V and complex-type β1,6-branched N-glycans in uveal and cutaneous melanoma cells.Acta Biochim Pol. 2015;62(2):323-8. doi: 10.18388/abp.2015_1050. Epub 2015 Jun 22. Acta Biochim Pol. 2015. PMID: 26098720
-
Expression of integrins α3β1 and α5β1 and GlcNAc β1,6 glycan branching influences metastatic melanoma cell migration on fibronectin.Eur J Cell Biol. 2013 Dec;92(12):355-62. doi: 10.1016/j.ejcb.2013.10.007. Epub 2013 Nov 1. Eur J Cell Biol. 2013. PMID: 24290991
-
Functional roles of the bisecting GlcNAc in integrin-mediated cell adhesion.Methods Enzymol. 2010;480:445-59. doi: 10.1016/S0076-6879(10)80019-9. Methods Enzymol. 2010. PMID: 20816221 Review.
-
Molecular role(s) for integrins in human melanoma invasion.Cancer Metastasis Rev. 1999;18(3):359-75. doi: 10.1023/a:1006317125454. Cancer Metastasis Rev. 1999. PMID: 10721490 Review.
Cited by
-
N-Glycosylation in progression of skin cancer.Med Oncol. 2019 Apr 29;36(6):50. doi: 10.1007/s12032-019-1270-4. Med Oncol. 2019. PMID: 31037368 Review.
-
Proteomic Analysis Reveals Salicylic Acid as a Pivotal Signal Molecule in Rice Response to Blast Disease Infection.Plants (Basel). 2022 Jun 27;11(13):1702. doi: 10.3390/plants11131702. Plants (Basel). 2022. PMID: 35807653 Free PMC article.
-
Hexosamine Biosynthetic Pathway and Glycosylation Regulate Cell Migration in Melanoma Cells.Front Oncol. 2019 Mar 5;9:116. doi: 10.3389/fonc.2019.00116. eCollection 2019. Front Oncol. 2019. PMID: 30891426 Free PMC article.
-
The glycomic effect of N-acetylglucosaminyltransferase III overexpression in metastatic melanoma cells. GnT-III modifies highly branched N-glycans.Glycoconj J. 2018 Apr;35(2):217-231. doi: 10.1007/s10719-018-9814-y. Epub 2018 Mar 3. Glycoconj J. 2018. PMID: 29502191 Free PMC article.
-
GOLPH3 Regulates EGFR in T98G Glioblastoma Cells by Modulating Its Glycosylation and Ubiquitylation.Int J Mol Sci. 2020 Nov 23;21(22):8880. doi: 10.3390/ijms21228880. Int J Mol Sci. 2020. PMID: 33238647 Free PMC article.
References
-
- Singh AD, Topham A. Survival rates with uveal melanoma in the United States: 1973–1997. Ophthalmology. 2003;110:962–5. - PubMed
-
- McLean IW. The biology of haematogenous metastasis in human uveal malignant melanoma. Virchows Arch A Pathol Anat Histopathol. 1993;422:433–7. - PubMed
-
- Shields JA, Shields CL, Donoso LA. Management of posterior uveal melanoma. Surv Ophthalmol. 1991;36:161–95. - PubMed
-
- De Croock L, Verbraeken H. Metastatic uveal melanoma: diagnosis and treatment: a literature review. Bull Soc Belge Ophtalmol. 2002;286:59–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
