Peroxiredoxin 6 in human brain: molecular forms, cellular distribution and association with Alzheimer's disease pathology
- PMID: 18386021
- PMCID: PMC2386158
- DOI: 10.1007/s00401-008-0373-3
Peroxiredoxin 6 in human brain: molecular forms, cellular distribution and association with Alzheimer's disease pathology
Abstract
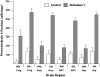
Peroxiredoxin 6 is an antioxidant enzyme and is the 1-cys member of the peroxiredoxin family. Using two-dimensional electrophoresis and Western blotting, we have shown for the first time that, in human control and brain tissue of patient's with Alzheimer's disease (AD), this enzyme exists as three major and five minor forms with pIs from 5.3 to 6.1. Using specific cellular markers, we have shown that peroxiredoxin 6 is present in astrocytes with very low levels in neurons, but not detectable in microglia or oligodendrocytes. In control brains, there was a very low level of peroxiredoxin 6 staining in astrocytes that was confined to a "halo" around the nucleus. In AD, there were marked increases in the number and staining intensity of peroxiredoxin 6 positive astrocytes in both gray and white matter in the midfrontal cortex, cingulate, hippocampus and amygdala. Confocal microscopy using antibodies to A beta peptide, tau and peroxiredoxin 6 showed that peroxiredoxin 6 positive astrocytes are closely involved with diffuse plaques and to a lesser extent with neuritic plaques, suggesting that plaques are producing reactive oxygen species. There appeared to be little astrocytic response to tau containing neurons. Although peroxiredoxin 6 positive astrocytes were seen to make multiple contacts with tau positive neurons, there was no intraneuronal colocalization. In brain tissue of patients with AD, many blood vessels exhibited peroxiredoxin 6 staining that appeared to be due to the astrocytic foot processes. These results suggest that oxidative stress conditions exist in AD and that peroxiredoxin 6 is an important antioxidant enzyme in human brain defenses.
Figures






References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1074/jbc.275.11.7918', 'is_inner': False, 'url': 'https://doi.org/10.1074/jbc.275.11.7918'}, {'type': 'PubMed', 'value': '10713108', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10713108/'}]}
- Akama KT, Van Eldik LJ (2000) β-amyloid stimulation of inducible nitric oxide synthetase in astrocytes is interleukin-1β and tumour necrosis factor-α (TNFα)-dependent, and involves a TNFα receptor-associated factor and NKFκB-inducing kinase-dependent signalling mechanism. J Biol Chem 275:7918–7924 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1046/j.1471-4159.2000.0751438.x', 'is_inner': False, 'url': 'https://doi.org/10.1046/j.1471-4159.2000.0751438.x'}, {'type': 'PubMed', 'value': '10987823', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/10987823/'}]}
- Barkats M, Millecamps S, Abrioux P, Geoffroy MC, Mallet J (2000) Overexpression of glutathione peroxidase increases the resistance of neuronal cells to Aβ-mediated neurotoxicity. J Neurochem 75:1438–1446 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0891-5849(02)00780-3', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0891-5849(02)00780-3'}, {'type': 'PubMed', 'value': '11978481', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11978481/'}]}
- Beal MF (2002) Oxidatively modified proteins in aging and disease. Free Radic Biol Med 32:797–803 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '1730616', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/1730616/'}]}
- Burdick D, Soreghan B, Kwon M, Kosmoski J, Knauer M, Henschen A, Yates J, Cotman C, Glabe C (1992) Assembly and aggregation properties of synthetic Alzheimer’s A4/β amyloid peptide analogs. J Biol Chem 267:546–554 - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0197-4580(01)00340-2', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0197-4580(01)00340-2'}, {'type': 'PubMed', 'value': '12392766', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12392766/'}]}
- Butterfield DA, Castegna A, Lauderback C M, Drake J (2002) Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol Aging 23:655–664 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

