PAX6 dosage effects on corneal development, growth, and wound healing
- PMID: 18386822
- PMCID: PMC2655055
- DOI: 10.1002/dvdy.21528
PAX6 dosage effects on corneal development, growth, and wound healing
Abstract
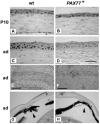
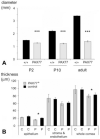
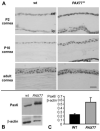
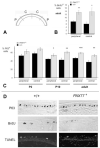
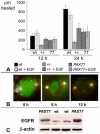
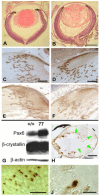
The requirement for correct dosage of the transcription factor Pax6 during corneal growth and development was investigated using the Pax6-overexpressing (PAX77) transgenic mouse. Transgenics had a microcornea phenotype due to failure of postnatal growth, associated with reduction in the number of cells layers in the corneal epithelium. Cell cycle progression was monitored using bromodeoxyuridine, p63, cyclin E, and phosphohistone-3 labeling: proliferation rates were higher in PAX77+ than wild-type, without a concomitant increase in apoptosis. Hence, failure of proliferation did not underlie microcornea. PAX77+ corneal epithelia had reduced levels of cytokeratin-12, and exhibited severe wound healing delay that, in contrast to Pax6+/- mice, could not be modulated by exogenous growth factors. PAX77+ lenses showed partial failure of lens fiber differentiation. The data demonstrate that anterior eye development is very sensitive to Pax6 dosage. Although there are similarities between the eye phenotype of Pax6 heterozygotes and overexpressing mice, there are also striking differences. Developmental
Figures

Similar articles
-
Effects of elevated Pax6 expression and genetic background on mouse eye development.Invest Ophthalmol Vis Sci. 2009 Sep;50(9):4045-59. doi: 10.1167/iovs.07-1630. Epub 2009 Apr 22. Invest Ophthalmol Vis Sci. 2009. PMID: 19387074 Free PMC article.
-
Effects of aberrant Pax6 gene dosage on mouse corneal pathophysiology and corneal epithelial homeostasis.PLoS One. 2011;6(12):e28895. doi: 10.1371/journal.pone.0028895. Epub 2011 Dec 29. PLoS One. 2011. PMID: 22220198 Free PMC article.
-
Interaction between hedgehog signalling and PAX6 dosage mediates maintenance and regeneration of the corneal epithelium.Mol Vis. 2012;18:139-50. Epub 2012 Jan 18. Mol Vis. 2012. PMID: 22275805 Free PMC article.
-
Interplay of Pax6 and SOX2 in lens development as a paradigm of genetic switch mechanisms for cell differentiation.Int J Dev Biol. 2004;48(8-9):819-27. doi: 10.1387/ijdb.041868hk. Int J Dev Biol. 2004. PMID: 15558474 Review.
-
Electrical signaling in control of ocular cell behaviors.Prog Retin Eye Res. 2012 Jan;31(1):65-88. doi: 10.1016/j.preteyeres.2011.10.001. Epub 2011 Oct 17. Prog Retin Eye Res. 2012. PMID: 22020127 Free PMC article. Review.
Cited by
-
The expression of Pax6 variants is subject to posttranscriptional regulation in the developing mouse eyelid.PLoS One. 2013;8(1):e53919. doi: 10.1371/journal.pone.0053919. Epub 2013 Jan 10. PLoS One. 2013. PMID: 23326536 Free PMC article.
-
Corneal epithelial development and homeostasis.Differentiation. 2023 Jul-Aug;132:4-14. doi: 10.1016/j.diff.2023.02.002. Epub 2023 Mar 1. Differentiation. 2023. PMID: 36870804 Free PMC article. Review.
-
Patient-derived cornea organoid model to study metabolomic characterization of rare disease: aniridia-associated keratopathy.BMC Ophthalmol. 2025 Jan 10;25(1):14. doi: 10.1186/s12886-024-03831-w. BMC Ophthalmol. 2025. PMID: 39794714 Free PMC article.
-
Effects of elevated Pax6 expression and genetic background on mouse eye development.Invest Ophthalmol Vis Sci. 2009 Sep;50(9):4045-59. doi: 10.1167/iovs.07-1630. Epub 2009 Apr 22. Invest Ophthalmol Vis Sci. 2009. PMID: 19387074 Free PMC article.
-
Establishing PAX6 as a biomarker to detect early loss of ocular phenotype in human patients with Sjögren's syndrome.Invest Ophthalmol Vis Sci. 2014 Sep 16;55(11):7079-84. doi: 10.1167/iovs.14-14828. Invest Ophthalmol Vis Sci. 2014. PMID: 25228544 Free PMC article.
References
-
- Aalfs CM, Fantes JA, Wenniger-Prick LJ, Sluijter S, Hennekam RC, van Heyningen V. Tandem duplication of 11p12-p13 in a child with borderline development delay and eye abnormalities: dose effect of the PAX6 gene product. Am J Med Genet. 1997;73:267–271. - PubMed
-
- Baulmann DC, Ohlmann A, Flugel-Koch C, Goswami S, Cvekl A, Tamm ER. Pax6 heterozygous eyes show defects in chamber angle differentiation that are associated with a wide spectrum of other anterior eye segment abnormalities. Mech Dev. 2002;118:3–17. - PubMed
-
- Chao LY, Huff V, Strong LC, Saunders GF. Mutation in the PAX6 gene in twenty patients with aniridia. Hum Mutat. 2000;15:332–339. - PubMed
-
- Chauhan BK, Reed NA, Zhang W, Duncan MK, Kilimann MW, Cvekl A. Identification of genes downstream of Pax6 in the mouse lens using cDNA microarrays. J Biol Chem. 2002a;277:11539–11548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

