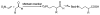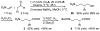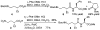Enantioselective organocatalytic Michael addition of aldehydes to nitroethylene: efficient access to gamma2-amino acids
- PMID: 18386925
- PMCID: PMC3429127
- DOI: 10.1021/ja800345r
Enantioselective organocatalytic Michael addition of aldehydes to nitroethylene: efficient access to gamma2-amino acids
Abstract
Enantioselective organocatalytic Michael addition of aldehydes to nitroethylene catalyzed by (S)-diphenylprolinol silyl ether provides beta-substituted-delta-nitroalcohols in nearly optically pure form (96-99% ee). The Michael adducts bear a single substituent adjacent to the carbonyl and can be efficiently converted to protected gamma2-amino acids, which are essential for the systematic conformational studies of gamma-peptide foldamers.
Similar articles
-
Peptide catalyzed asymmetric conjugate addition reactions of aldehydes to nitroethylene--a convenient entry into gamma2-amino acids.J Am Chem Soc. 2008 Apr 30;130(17):5610-1. doi: 10.1021/ja801027s. Epub 2008 Apr 4. J Am Chem Soc. 2008. PMID: 18386927
-
Enantioselective organocatalytic aminomethylation of aldehydes: a role for ionic interactions and efficient access to beta2-amino acids.J Am Chem Soc. 2006 May 31;128(21):6804-5. doi: 10.1021/ja061731n. J Am Chem Soc. 2006. PMID: 16719457 Free PMC article.
-
Direct enantioselective organocatalytic hydroxymethylation of aldehydes catalyzed by alpha,alpha-diphenylprolinol trimethylsilyl ether.Org Lett. 2009 Oct 15;11(20):4544-7. doi: 10.1021/ol9017479. Org Lett. 2009. PMID: 19757801
-
Organocatalytic asymmetric 1,4-addition of aldehydes to acridiniums catalyzed by a diarylprolinol silyl ether.J Org Chem. 2012 Apr 6;77(7):3583-8. doi: 10.1021/jo2025114. Epub 2012 Mar 19. J Org Chem. 2012. PMID: 22390361
-
Diphenylprolinol silyl ether as a catalyst in an asymmetric, catalytic, and direct michael reaction of nitroethanol with alpha,beta-unsaturated aldehydes.Org Lett. 2009 Sep 17;11(18):4056-9. doi: 10.1021/ol901483x. Org Lett. 2009. PMID: 19739683
Cited by
-
Asymmetric Michael Addition in Synthesis of β-Substituted GABA Derivatives.Molecules. 2022 Jun 13;27(12):3797. doi: 10.3390/molecules27123797. Molecules. 2022. PMID: 35744921 Free PMC article. Review.
-
Environmental Modulation of Chiral Prolinamide Catalysts for Stereodivergent Conjugate Addition.J Catal. 2022 Feb;406:126-133. doi: 10.1016/j.jcat.2022.01.003. Epub 2022 Jan 10. J Catal. 2022. PMID: 35087258 Free PMC article.
-
Crystal structure of N-[(1S,2S)-2-amino-cyclo-hex-yl]-2,4,6-tri-methyl-benzene-sulfonamide.Acta Crystallogr E Crystallogr Commun. 2015 Nov 21;71(Pt 12):1521-4. doi: 10.1107/S205698901502191X. eCollection 2015 Dec 1. Acta Crystallogr E Crystallogr Commun. 2015. PMID: 26870419 Free PMC article.
-
Iterative One-Carbon Homologation of Unmodified Carboxylic Acids.J Am Chem Soc. 2024 Dec 18;146(50):34285-34291. doi: 10.1021/jacs.4c13630. Epub 2024 Dec 10. J Am Chem Soc. 2024. PMID: 39656028 Free PMC article.
-
Stereospecific synthesis of conformationally constrained gamma-amino acids: new foldamer building blocks that support helical secondary structure.J Am Chem Soc. 2009 Nov 11;131(44):16018-20. doi: 10.1021/ja907233q. J Am Chem Soc. 2009. PMID: 19886693 Free PMC article.
References
-
- Perlmutter P. Conjugate Addition Reactions in Organic Synthesis. Pergamon Press; Oxford: 1992.
-
-
For recent reviews, see: Jarvo ER, Miller SJ. Tetrahedron. 2002;58:2481.Sibi MP, Manyem S. Tetrahedron. 2000;56:8033.Yamaguchi M. In: Comprehensive Asymmetric Catalysis I–III. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Chapter 31.2 Springer-Verlag; Berlin-Heidelberg, Germany: 1999.
-
-
-
For recent reviews, see: Berner OM, Tedeschi L, Enders D. Eur J Org Chem. 2002. p. 1877.Pellissier H. Tetrahedron. 2007;63:9267.Santanu M, Yang JW, Hoffmann S, List B. Chem Rev. 2007;107:5471.
-
-
-
For selected examples, see: Betancort JM, Barbas CF., III Org Lett. 2001;3:3737.Mase N, Thayumanavan R, Tanaka F, Barbas CF., III Org Lett. 2004;6:2527.Mase N, Watanabe K, Yoda H, Takabe K, Tanaka F, Barbas CF., III J Am Chem Soc. 2006;128:4966.Wang W, Wang J, Li H. Angew Chem, Int Ed. 2005;44:1369.Hayashi Y, Gotoh H, Hayashi T, Shoji M. Angew Chem, Int Ed. 2005;44:4212.Lalonde MP, Chen YG, Jacobsen EN. Angew Chem, Int Ed. 2005;45:6366.Wiesner M, Revell JD, Wennemers H. Angew Chem, Int Ed. 2008;47:1871.
-
-
-
For selected examples, see: List B, Pojarliev P, Martin HJ. Org Lett. 2001;3:2423.Okino T, Hoashi Y, Takemoto Y. J Am Chem Soc. 2003;125:12672.Xu Y, Córdova A. Chem Commun. 2006:460.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources