Role of adenomatous polyposis coli (APC) and microtubules in directional cell migration and neuronal polarization
- PMID: 18387324
- PMCID: PMC2673958
- DOI: 10.1016/j.semcdb.2008.02.003
Role of adenomatous polyposis coli (APC) and microtubules in directional cell migration and neuronal polarization
Abstract
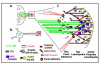
In response to extracellular signals during embryonic development, cells undergo directional movements to specific sites and establish proper connections to other cells to form organs and tissues. Cell extension and migration in the direction of extracellular cues is mediated by the actin and microtubule cytoskeletons, and recent results have shed new light on how these pathways are activated by neurotrophins, Wnt or extracellular matrix. These signals lead to modifications of microtubule-associated proteins (MAPs) and point to glycogen synthase kinase (GSK) 3beta as a key regulator of microtubule function during directional migration. This review will summarize these results and then focus on the role of microtubule-binding protein adenomatous polyposis coli (APC) in neuronal polarization and directed migration, and on its regulation by GSK3beta.
Figures


References
-
- Thiery JP. Mechanisms of cell migration in the vertebrate embryo. Cell Differ. 1984;15:1–15. - PubMed
-
- Hynes RO, Lander AD. Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992;68:303–22. - PubMed
-
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. - PubMed
-
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–33. - PubMed
-
- Lykissas MG, Batistatou AK, Charalabopoulos KA, Beris AE. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr Neurovasc Res. 2007;4:143–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

