The effect of donor age on corneal transplantation outcome results of the cornea donor study
- PMID: 18387407
- PMCID: PMC2810523
- DOI: 10.1016/j.ophtha.2008.01.003
The effect of donor age on corneal transplantation outcome results of the cornea donor study
Abstract
Objective: To determine whether graft survival over a 5-year follow-up period using corneal tissue from donors older than 65 is similar to graft survival using corneas from younger donors.
Design: Multicenter prospective, double-masked, controlled clinical trial.
Participants: One thousand ninety subjects undergoing corneal transplantation for a moderate-risk condition (principally Fuchs' dystrophy or pseudophakic corneal edema); 11 subjects with ineligible diagnoses were not included.
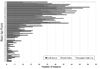
Methods: Forty-three participating eye banks provided corneas from donors in the age range of 12 to 75 with endothelial cell densities of 2300 to 3300 cells/mm(2), using a random approach without respect to recipient factors. The 105 participating surgeons at 80 sites were masked to information about the donor cornea including donor age. Surgery and postoperative care were performed according to the surgeons' usual routines. Subjects were observed for 5 years.
Main outcome measures: Graft failure, defined as a regraft or a cloudy cornea that was sufficiently opaque as to compromise vision for a minimum of 3 consecutive months.
Results: The 5-year cumulative probability of graft survival was 86% in both the <66.0 donor age group and the >/=66.0 donor age group (difference = 0%, upper limit of 1-sided 95% confidence interval = 4%). In a statistical model with donor age as a continuous variable, there was no significant relationship between donor age and outcome (P = 0.11). Three graft failures were due to primary donor failure, 8 to uncorrectable refractive error, 48 to graft rejection, 46 to endothelial decompensation (23 of which had a prior, resolved episode of probable or definite graft rejection), and 30 to other causes. Distributions of the causes of graft failure did not differ between donor age groups.
Conclusions: Five-year graft survivals for cornea transplants at moderate risk for failure are similar using corneas from donors >/= 66.0 years and donors < 66.0. Surgeons and patients now have evidence that corneas comparable in quality to those used in this study from donors through age 75 are suitable for transplantation.
Conflict of interest statement
Figures


References
-
- Eye Bank Association of America. Washington, DC: EBAA; 2006. 2006 Eye Banking Statistical Report.
-
- Human Cells, Tissues, and Cellular and Tissue-Based Products; Donor Screening and Testing, and Related Labeling, Final Rule. Federal Register. 2007 Jun 19;72:33667–33669. - PubMed
-
- Cavanagh HD. Eye Banking 1995: danger and opportunity. Cornea. 1995;14:545–546. - PubMed
-
- Chu W, Dahl P, O'Neill MJ. Benefits of specular microscopy in evaluating eye donors aged 66 and older. Cornea. 1995;14:568–570. 634. - PubMed
-
- Mattern RM, Heck EL, Cavanagh HD. The impact on tissue utilization of screening donor corneas by specular microscopy at the University of Texas Southwestern Medical Center. Cornea. 1995;14:562–567. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

