Intrathecal rosiglitazone acts at peroxisome proliferator-activated receptor-gamma to rapidly inhibit neuropathic pain in rats
- PMID: 18387855
- PMCID: PMC2556259
- DOI: 10.1016/j.jpain.2008.02.002
Intrathecal rosiglitazone acts at peroxisome proliferator-activated receptor-gamma to rapidly inhibit neuropathic pain in rats
Abstract
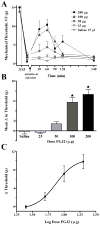
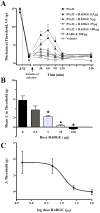
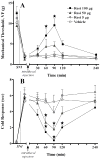
In this report, we demonstrate the transcription, expression, and DNA-binding properties of the peroxisome proliferator-activated receptor (PPAR)-gamma subtype of the peroxisome proliferator-activated nuclear receptor family to the spinal cord with real-time PCR, Western blot, and electrophoretic mobility shift assay. To test the hypothesis that activation of spinal PPAR-gamma decreases nerve injury-induced allodynia, we intrathecally administered PPAR-gamma agonists and/or antagonists in rats after transection of the tibial and common peroneal branches of the sciatic nerve. Single injection of either a natural (15-deoxy-prostaglandin J2, 15d-PGJ2) or synthetic (rosiglitazone) PPAR-gamma agonist dose-dependently decreased mechanical and cold hypersensitivity. These effects were maximal at a dose of 100 microg and peaked at approximately 60 minutes after injection, a rapid time course suggestive of transcription-independent mechanisms of action. Concurrent administration of a PPAR-gamma antagonist (bisphenol A diglycidyl ether, BADGE) reversed the effects of 15d-PGJ2 and rosiglitazone, further indicating a receptor-mediated effect. In animals without nerve injury, rosiglitazone did not alter motor coordination, von Frey threshold, or withdrawal response to a cool stimulus. Intraperitoneal and intracerebroventricular administration of PPAR-gamma agonists (100 microg) did not decrease mechanical and cold hypersensitivity, arguing against effects subsequent to diffusion from the intrathecal space. We conclude that ligand-induced activation of spinal PPAR-gamma rapidly reverses nerve injury-induced mechanical allodynia. New or currently available drugs targeted at spinal PPAR-gamma may yield important therapeutic effects for the management of neuropathic pain.
Perspective: PPAR-gamma receptor agonists such as rosiglitazone and pioglitazone are approved as insulin sensitizers by the United States Food and Drug Administration. We demonstrate PPAR-gamma expression in the spinal cord and report that activation of these receptors inhibits allodynia. BBB-permeant PPAR-gamma agonists may yield important therapeutic effects for the management of neuropathic pain.
Figures




Similar articles
-
Rosiglitazone and 15-deoxy-Delta12,14-prostaglandin J2, ligands of the peroxisome proliferator-activated receptor-gamma (PPAR-gamma), reduce ischaemia/reperfusion injury of the gut.Br J Pharmacol. 2003 Sep;140(2):366-76. doi: 10.1038/sj.bjp.0705419. Epub 2003 Aug 11. Br J Pharmacol. 2003. PMID: 12970094 Free PMC article.
-
Activation of peroxisome proliferator-activated receptor gamma in brain inhibits inflammatory pain, dorsal horn expression of Fos, and local edema.Neuropharmacology. 2010 Feb;58(2):337-45. doi: 10.1016/j.neuropharm.2009.10.008. Epub 2009 Nov 3. Neuropharmacology. 2010. PMID: 19891980 Free PMC article.
-
Agonists at PPAR-gamma suppress angiotensin II-induced production of plasminogen activator inhibitor-1 and extracellular matrix in rat cardiac fibroblasts.Br J Pharmacol. 2008 Apr;153(7):1409-19. doi: 10.1038/bjp.2008.21. Epub 2008 Feb 18. Br J Pharmacol. 2008. PMID: 18278065 Free PMC article.
-
Building and Testing PPARγ Therapeutic ELB00824 with an Improved Therapeutic Window for Neuropathic Pain.Molecules. 2020 Mar 3;25(5):1120. doi: 10.3390/molecules25051120. Molecules. 2020. PMID: 32138198 Free PMC article. Review.
-
Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists.Front Biosci. 2008 Jan 1;13:1813-26. doi: 10.2741/2802. Front Biosci. 2008. PMID: 17981670 Free PMC article. Review.
Cited by
-
Sustained relief of trigeminal neuropathic pain by a blood-brain barrier penetrable PPAR gamma agonist.Mol Pain. 2019 Jan-Dec;15:1744806919884498. doi: 10.1177/1744806919884498. Mol Pain. 2019. PMID: 31588847 Free PMC article.
-
The PPARγ agonist pioglitazone produces a female-predominant inhibition of hyperalgesia associated with surgical incision, peripheral nerve injury, and painful diabetic neuropathy.Neuropharmacology. 2022 Mar 1;205:108907. doi: 10.1016/j.neuropharm.2021.108907. Epub 2021 Nov 29. Neuropharmacology. 2022. PMID: 34856203 Free PMC article.
-
Peroxisome proliferator activated receptor-gamma (PPAR-γ) ligand, pioglitazone, increases analgesic and anti-inflammatory effects of naproxen.Naunyn Schmiedebergs Arch Pharmacol. 2024 Mar;397(3):1633-1646. doi: 10.1007/s00210-023-02715-y. Epub 2023 Sep 12. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37698622
-
Zerumbone Ameliorates Neuropathic Pain Symptoms via Cannabinoid and PPAR Receptors Using In Vivo and In Silico Models.Molecules. 2021 Jun 24;26(13):3849. doi: 10.3390/molecules26133849. Molecules. 2021. PMID: 34202590 Free PMC article.
-
Effects of long-term pioglitazone treatment on peripheral and central markers of aging.PLoS One. 2010 Apr 29;5(4):e10405. doi: 10.1371/journal.pone.0010405. PLoS One. 2010. PMID: 20454453 Free PMC article.
References
-
- Abdelrahman M, Sivarajah A, Thiemermann C. Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury, inflammation and shock. Cardiovasc Res. 2005;65:772–81. - PubMed
-
- Berger J, Akiyama T, Meinke P. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–51. - PubMed
-
- Bernardo A, M L. PPAR-gamma agonists as regulators of microglial activation and brain inflammation. Curr Pharm Des. 2006;12:93–109. - PubMed
-
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 1994;53:55–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

