Phorbol 12-myristate 13-acetate-dependent protein kinase C delta-Tyr311 phosphorylation in cardiomyocyte caveolae
- PMID: 18387943
- PMCID: PMC2440626
- DOI: 10.1074/jbc.M800333200
Phorbol 12-myristate 13-acetate-dependent protein kinase C delta-Tyr311 phosphorylation in cardiomyocyte caveolae
Abstract
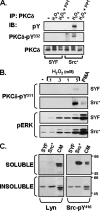
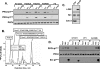
Protein kinase Cdelta (PKCdelta) activation is generally attributed to lipid cofactor-dependent allosteric activation mechanisms at membranes. However, recent studies indicate that PKCdelta also is dynamically regulated through tyrosine phosphorylation in H(2)O(2)- and phorbol 12-myristate 13-acetate (PMA)-treated cardiomyocytes. H(2)O(2) activates Src and related Src-family kinases (SFKs), which function as dual PKCdelta-Tyr(311) and -Tyr(332) kinases in vitro and contribute to H(2)O(2)-dependent PKCdelta-Tyr(311)/Tyr(332) phosphorylation in cardiomyocytes and in mouse embryo fibroblasts. H(2)O(2)-dependent PKCdelta-Tyr(311)/Tyr(332) phosphorylation is defective in SYF cells (deficient in SFKs) and restored by Src re-expression. PMA also promotes PKCdelta-Tyr(311) phosphorylation, but this is not associated with SFK activation or PKCdelta-Tyr(332) phosphorylation. Rather, PMA increases PKCdelta-Tyr(311) phosphorylation by delivering PKCdelta to SFK-enriched caveolae. Cyclodextrin treatment disrupts caveolae and blocks PMA-dependent PKCdelta-Tyr(311) phosphorylation, without blocking H(2)O(2)-dependent PKCdelta-Tyr(311) phosphorylation. The enzyme that acts as a PKCdelta-Tyr(311) kinase without increasing PKCdelta phosphorylation at Tyr(332) in PMA-treated cardiomyocytes is uncertain. Although in vitro kinase assays implicate c-Abl as a selective PKCdelta-Tyr(311) kinase, PMA-dependent PKCdelta-Tyr(311) phosphorylation persists in cardiomyocytes treated with the c-Abl inhibitor ST1571 and c-Abl is not detected in caveolae; these results effectively exclude a c-Abl-dependent process. Finally, we show that 1,2-dioleoyl-sn-glycerol mimics the effect of PMA to drive PKCdelta to caveolae and increase PKCdelta-Tyr(311) phosphorylation, whereas G protein-coupled receptor agonists such as norepinephrine and endothelin-1 do not. These results suggest that norepinephrine and endothelin-1 increase 1,2-dioleoyl-sn-glycerol accumulation and activate PKCdelta exclusively in non-caveolae membranes. Collectively, these results identify stimulus-specific PKCdelta localization and tyrosine phosphorylation mechanisms that could be targeted for therapeutic advantage.
Figures



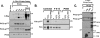

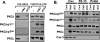
Similar articles
-
Protein kinase Cepsilon (PKCepsilon) and Src control PKCdelta activation loop phosphorylation in cardiomyocytes.J Biol Chem. 2007 Aug 10;282(32):23631-8. doi: 10.1074/jbc.M701676200. Epub 2007 Jun 14. J Biol Chem. 2007. PMID: 17569658 Free PMC article.
-
Stimulus-specific differences in protein kinase C delta localization and activation mechanisms in cardiomyocytes.J Biol Chem. 2004 Apr 30;279(18):19350-61. doi: 10.1074/jbc.M311096200. Epub 2004 Feb 17. J Biol Chem. 2004. PMID: 14970215
-
Phorbol 12-myristate 13-acetate induces epidermal growth factor receptor transactivation via protein kinase Cdelta/c-Src pathways in glioblastoma cells.J Biol Chem. 2005 Mar 4;280(9):7729-38. doi: 10.1074/jbc.M409056200. Epub 2004 Dec 23. J Biol Chem. 2005. PMID: 15618223 Free PMC article.
-
Distinctive activation mechanisms and functions for protein kinase Cdelta.Biochem J. 2004 Dec 15;384(Pt 3):449-59. doi: 10.1042/BJ20040704. Biochem J. 2004. PMID: 15491280 Free PMC article. Review.
-
Regulation of phospholamban and troponin-I phosphorylation in the intact rat cardiomyocytes by adrenergic and cholinergic stimuli: roles of cyclic nucleotides, calcium, protein kinases and phosphatases and depolarization.Mol Cell Biochem. 1995 Aug-Sep;149-150:103-26. doi: 10.1007/BF01076569. Mol Cell Biochem. 1995. PMID: 8569720 Review.
Cited by
-
Cardiomyocytes Sense Matrix Rigidity through a Combination of Muscle and Non-muscle Myosin Contractions.Dev Cell. 2018 Feb 5;44(3):326-336.e3. doi: 10.1016/j.devcel.2017.12.024. Epub 2018 Jan 26. Dev Cell. 2018. PMID: 29396114 Free PMC article.
-
The C2 Domain and Altered ATP-Binding Loop Phosphorylation at Ser³⁵⁹ Mediate the Redox-Dependent Increase in Protein Kinase C-δ Activity.Mol Cell Biol. 2015 May;35(10):1727-40. doi: 10.1128/MCB.01436-14. Epub 2015 Mar 9. Mol Cell Biol. 2015. PMID: 25755284 Free PMC article.
-
Identification and characterisation of lamprey protein kinase C delta-like gene.Sci Rep. 2017 Sep 22;7(1):12214. doi: 10.1038/s41598-017-12526-w. Sci Rep. 2017. PMID: 28939820 Free PMC article.
-
Regulatory autophosphorylation sites on protein kinase C-delta at threonine-141 and threonine-295.Biochemistry. 2009 Jun 2;48(21):4642-51. doi: 10.1021/bi802171c. Biochemistry. 2009. PMID: 19366211 Free PMC article.
-
PTEN protein phosphatase activity regulates metastasis by targeting PKCδ.iScience. 2025 May 26;28(6):112741. doi: 10.1016/j.isci.2025.112741. eCollection 2025 Jun 20. iScience. 2025. PMID: 40546936 Free PMC article.
References
-
- Rybin, V. O., Sabri, A., Short, J., Braz, J. C., Molkentin, J. D., and Steinberg, S. F. (2003) J. Biol. Chem. 278 14555–14564 - PubMed
-
- Le Good, J. A., Ziegler, W. H., Parekh, D. B., Alessi, D. R., Cohen, P., and Parker, P. J. (1998) Science 281 2042–2045 - PubMed
-
- Stempka, L., Schnolzer, M., Radke, S., Rincke, G., Marks, F., and Gschwendt, M. (1999) J. Biol. Chem. 274 8886–8892 - PubMed
-
- Liu, Y., Belkina, N. V., Graham, C., and Shaw, S. (2006) J. Biol. Chem. 281 12102–12111 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

