Phorbol 12-myristate 13-acetate-dependent protein kinase C delta-Tyr311 phosphorylation in cardiomyocyte caveolae
- PMID: 18387943
- PMCID: PMC2440626
- DOI: 10.1074/jbc.M800333200
Phorbol 12-myristate 13-acetate-dependent protein kinase C delta-Tyr311 phosphorylation in cardiomyocyte caveolae
Abstract
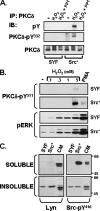
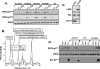
Protein kinase Cdelta (PKCdelta) activation is generally attributed to lipid cofactor-dependent allosteric activation mechanisms at membranes. However, recent studies indicate that PKCdelta also is dynamically regulated through tyrosine phosphorylation in H(2)O(2)- and phorbol 12-myristate 13-acetate (PMA)-treated cardiomyocytes. H(2)O(2) activates Src and related Src-family kinases (SFKs), which function as dual PKCdelta-Tyr(311) and -Tyr(332) kinases in vitro and contribute to H(2)O(2)-dependent PKCdelta-Tyr(311)/Tyr(332) phosphorylation in cardiomyocytes and in mouse embryo fibroblasts. H(2)O(2)-dependent PKCdelta-Tyr(311)/Tyr(332) phosphorylation is defective in SYF cells (deficient in SFKs) and restored by Src re-expression. PMA also promotes PKCdelta-Tyr(311) phosphorylation, but this is not associated with SFK activation or PKCdelta-Tyr(332) phosphorylation. Rather, PMA increases PKCdelta-Tyr(311) phosphorylation by delivering PKCdelta to SFK-enriched caveolae. Cyclodextrin treatment disrupts caveolae and blocks PMA-dependent PKCdelta-Tyr(311) phosphorylation, without blocking H(2)O(2)-dependent PKCdelta-Tyr(311) phosphorylation. The enzyme that acts as a PKCdelta-Tyr(311) kinase without increasing PKCdelta phosphorylation at Tyr(332) in PMA-treated cardiomyocytes is uncertain. Although in vitro kinase assays implicate c-Abl as a selective PKCdelta-Tyr(311) kinase, PMA-dependent PKCdelta-Tyr(311) phosphorylation persists in cardiomyocytes treated with the c-Abl inhibitor ST1571 and c-Abl is not detected in caveolae; these results effectively exclude a c-Abl-dependent process. Finally, we show that 1,2-dioleoyl-sn-glycerol mimics the effect of PMA to drive PKCdelta to caveolae and increase PKCdelta-Tyr(311) phosphorylation, whereas G protein-coupled receptor agonists such as norepinephrine and endothelin-1 do not. These results suggest that norepinephrine and endothelin-1 increase 1,2-dioleoyl-sn-glycerol accumulation and activate PKCdelta exclusively in non-caveolae membranes. Collectively, these results identify stimulus-specific PKCdelta localization and tyrosine phosphorylation mechanisms that could be targeted for therapeutic advantage.
Figures



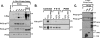

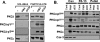
References
-
- Rybin, V. O., Sabri, A., Short, J., Braz, J. C., Molkentin, J. D., and Steinberg, S. F. (2003) J. Biol. Chem. 278 14555–14564 - PubMed
-
- Le Good, J. A., Ziegler, W. H., Parekh, D. B., Alessi, D. R., Cohen, P., and Parker, P. J. (1998) Science 281 2042–2045 - PubMed
-
- Stempka, L., Schnolzer, M., Radke, S., Rincke, G., Marks, F., and Gschwendt, M. (1999) J. Biol. Chem. 274 8886–8892 - PubMed
-
- Liu, Y., Belkina, N. V., Graham, C., and Shaw, S. (2006) J. Biol. Chem. 281 12102–12111 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

