Stochasticity and cell fate
- PMID: 18388284
- PMCID: PMC2605794
- DOI: 10.1126/science.1147888
Stochasticity and cell fate
Abstract
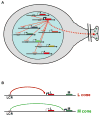
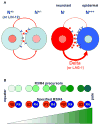
Fundamental to living cells is the capacity to differentiate into subtypes with specialized attributes. Understanding the way cells acquire their fates is a major challenge in developmental biology. How cells adopt a particular fate is usually thought of as being deterministic, and in the large majority of cases it is. That is, cells acquire their fate by virtue of their lineage or their proximity to an inductive signal from another cell. In some cases, however, and in organisms ranging from bacteria to humans, cells choose one or another pathway of differentiation stochastically, without apparent regard to environment or history. Stochasticity has important mechanistic requirements. We speculate on why stochasticity is advantageous-and even critical in some circumstances-to the individual, the colony, or the species.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

