Recycling of IRAP from the plasma membrane back to the insulin-responsive compartment requires the Q-SNARE syntaxin 6 but not the GGA clathrin adaptors
- PMID: 18388312
- PMCID: PMC4327992
- DOI: 10.1242/jcs.017517
Recycling of IRAP from the plasma membrane back to the insulin-responsive compartment requires the Q-SNARE syntaxin 6 but not the GGA clathrin adaptors
Erratum in
- J Cell Sci. 2008 Jul 1;121(Pt 13):2275. Hou, June C [added]
Abstract
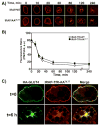
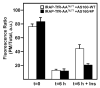
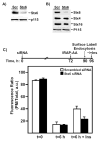
Insulin recruits two transmembrane proteins, GLUT4 and IRAP, to the plasma membrane of muscle cells and adipocytes. The subcellular trafficking and localization of GLUT4, and to a lesser extent IRAP, have been intensely studied, yet the molecular mechanisms responsible for their insulin-responsive compartmentalization remain unknown. Herein we have investigated the endocytosis and recycling of IRAP from the cell surface back to the insulin-responsive compartment (IRC). Our results show that a key dileucine motif at position 76,77 (LL76,77), although required for the initial biosynthetic entry of IRAP into the IRC, is dispensable for entry into the IRC via the endosomal system. Indeed, we found that an AA76,77 mutant of IRAP is fully capable of undergoing endocytosis and is correctly routed back to the IRC. To verify that the AA76,77 mutant enters the bona fide IRC, we show that the internalized IRAP-AA76,77 construct is sequestered in an IRC that is insensitive to brefeldin A yet sensitive to a dominant-interfering mutant of AS160 (AS160-4P). In addition, we show that the GGA clathrin adaptors are not required for the re-entry of IRAP from the cell surface back into the IRC, whereas the Q-SNARE syntaxin 6 is required for this process.
Figures




Similar articles
-
A specific dileucine motif is required for the GGA-dependent entry of newly synthesized insulin-responsive aminopeptidase into the insulin-responsive compartment.J Biol Chem. 2006 Nov 3;281(44):33457-66. doi: 10.1074/jbc.M601583200. Epub 2006 Aug 31. J Biol Chem. 2006. PMID: 16945927
-
Insulin-responsive aminopeptidase trafficking in 3T3-L1 adipocytes.J Biol Chem. 2000 Jan 28;275(4):2560-7. doi: 10.1074/jbc.275.4.2560. J Biol Chem. 2000. PMID: 10644714
-
Initial entry of IRAP into the insulin-responsive storage compartment occurs prior to basal or insulin-stimulated plasma membrane recycling.Am J Physiol Endocrinol Metab. 2005 Nov;289(5):E746-52. doi: 10.1152/ajpendo.00175.2005. Epub 2005 May 31. Am J Physiol Endocrinol Metab. 2005. PMID: 15928022
-
The sugar is sIRVed: sorting Glut4 and its fellow travelers.Traffic. 2011 Jun;12(6):665-71. doi: 10.1111/j.1600-0854.2011.01175.x. Epub 2011 Mar 15. Traffic. 2011. PMID: 21306486 Review.
-
The insulin-regulated aminopeptidase: a companion and regulator of GLUT4.Front Biosci. 2003 May 1;8:s410-20. doi: 10.2741/1078. Front Biosci. 2003. PMID: 12700100 Review.
Cited by
-
Sorting of GLUT4 into its insulin-sensitive store requires the Sec1/Munc18 protein mVps45.Mol Biol Cell. 2013 Aug;24(15):2389-97. doi: 10.1091/mbc.E13-01-0011. Epub 2013 Jun 5. Mol Biol Cell. 2013. PMID: 23741049 Free PMC article.
-
Regulation of intracellular membrane trafficking and cell dynamics by syntaxin-6.Biosci Rep. 2012 Aug;32(4):383-91. doi: 10.1042/BSR20120006. Biosci Rep. 2012. PMID: 22489884 Free PMC article. Review.
-
Biogenesis and regulation of insulin-responsive vesicles containing GLUT4.Curr Opin Cell Biol. 2010 Aug;22(4):506-12. doi: 10.1016/j.ceb.2010.03.012. Epub 2010 Apr 21. Curr Opin Cell Biol. 2010. PMID: 20417083 Free PMC article. Review.
-
EARP is a multisubunit tethering complex involved in endocytic recycling.Nat Cell Biol. 2015 May;17(5):639-50. doi: 10.1038/ncb3129. Epub 2015 Mar 23. Nat Cell Biol. 2015. PMID: 25799061 Free PMC article.
-
Syntaxin 6 and CAL mediate the degradation of the cystic fibrosis transmembrane conductance regulator.Mol Biol Cell. 2010 Apr 1;21(7):1178-87. doi: 10.1091/mbc.e09-03-0229. Epub 2010 Feb 3. Mol Biol Cell. 2010. PMID: 20130090 Free PMC article.
References
-
- Abel ED, Graveleau C, Betuing S, Pham M, Reay PA, Kandror V, Kupriyanova T, Xu Z, Kandror KV. Regulation of insulin-responsive aminopeptidase expression and targeting in the insulin-responsive vesicle compartment of glucose transporter isoform 4-deficient cardiomyocytes. Mol Endocrinol. 2004;18:2491–2501. - PubMed
-
- Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2007;292:E1191–E1200. - PubMed
-
- Bao S, Smith RM, Jarett L, Garvey WT. The effects of brefeldin A on the glucose transport system in rat adipocytes. Implications regarding the intracellular locus of insulin-sensitive Glut4. J Biol Chem. 1995;270:30199–30204. - PubMed
-
- Bock JB, Lin RC, Scheller RH. A new syntaxin family member implicated in targeting of intracellular transport vesicles. J Biol Chem. 1996;271:17961–17965. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

