Heterologous boosting of recombinant adenoviral prime immunization with a novel vesicular stomatitis virus-vectored tuberculosis vaccine
- PMID: 18388911
- PMCID: PMC7185538
- DOI: 10.1038/mt.2008.59
Heterologous boosting of recombinant adenoviral prime immunization with a novel vesicular stomatitis virus-vectored tuberculosis vaccine
Abstract
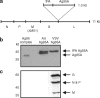
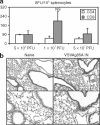
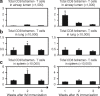
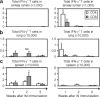
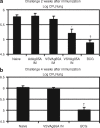
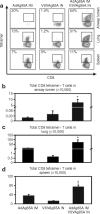
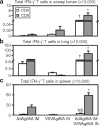
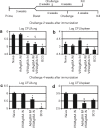
Pulmonary tuberculosis (TB) remains a serious health problem worldwide. Effective vaccination strategies are needed. We report the development of a novel TB vaccine using vesicular stomatitis virus (VSV) as a viral vector system to express Ag85A. VSVAg85A was shown to be immunogenic when given to mice by either an intranasal or an intramuscular (i.m.) route. Although distinct T-cell profiles resulted from both routes of immunization, only intranasal delivery generated a mucosal T-cell response that was protective upon pulmonary Mycobacterium tuberculosis (M.tb) challenge. While this protection manifested at an early time-point after immunization, it was not sustained. The potential of VSVAg85A to be used as a mucosal booster for parenteral priming by an adenoviral TB vaccine expressing Ag85A (AdAg85A) was investigated. VSVAg85A immunization markedly boosted antigen-specific T-cell responses in the airway lumen while also augmenting immune activation in the systemic compartment, after AdAg85A priming. This translated into significantly better protective efficacy against pulmonary challenge with M.tb than either vaccine used alone. Our study therefore suggests that VSV as a vector system is a promising candidate to be used in a heterologous viral prime-boost immunization regimen against intracellular bacterial infection.
Figures
References
-
- World Health Organization . Tuberculosis facts. World Health Organization; Geneva: 2007.
-
- Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. - PubMed
-
- Andersen P, Doherty TM. The success and failure of BCG—implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3:656–662. - PubMed
-
- World Health Organization BCG vaccine. WHO position paper. Wkly Epidemiol Rec. 2004;79:27–38. - PubMed
-
- Brewer TF. Preventing tuberculosis with bacillus Calmette-Guérin vaccine: a meta-analysis of the literature. Clin Infect Dis. 2000;31(suppl. 3):S64–S67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

