Extended transgene expression from a nonintegrating adenoviral vector containing retroviral elements
- PMID: 18388914
- PMCID: PMC5264529
- DOI: 10.1038/mt.2008.56
Extended transgene expression from a nonintegrating adenoviral vector containing retroviral elements
Abstract
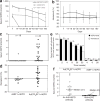
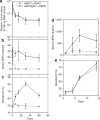
We studied the effects of specific retroviral elements in a first-generation serotype 5 adenoviral (Ad5) vector, AdLTR(2)EF1alpha-hEPO. This vector contains 858 base pair (bp) [251-bp envelope sequence plus 607-bp long-terminal repeat (LTR)] from Moloney murine leukemia virus (MoMLV), upstream of the human elongation factor-1alpha (EF1alpha) promoter and human erythropoietin (hEPO) cDNA, with the LTR sequence downstream of the polyadenylation signal. We compared expression of AdLTR(2)EF1alpha-hEPO with corresponding expressions of two conventional Ad5 vectors, AdEF1alpha-hEPO and AdCMV-hEPO, in vivo in submandibular glands in rats. Both the conventional vectors yielded low serum hEPO levels by day 7, and little change in hematocrits. In contrast, after receiving AdLTR(2)EF1alpha-hEPO, the rats showed elevated hEPO levels and hematocrits for 1-3 months. In vitro studies showed that the integration efficiencies of all the vectors were similar (approximately 10(-3)). Approximately 0.1% of the vector genomes were present 1 year after delivery in the case of each of the three vectors, primarily as intact linear double-strand DNA. The unique results seen with AdLTR(2)EF1alpha-hEPO are partly because of LTR enhancer activity. However, other cis-acting activity, which is not immunomodulatory but nevertheless influences promoter methylation, appears to be involved. A vector such as AdLTR(2)EF1alpha-hEPO may prove useful in clinical applications in which extended, but not "permanent," transgene expression is desirable.
Figures




References
-
- Schaack J, Ho WY, Freimuth P, Shenk T. Adenovirus terminal protein mediates both nuclear matrix association and efficient transcription of adenovirus DNA. Genes Dev. 1990;4:1197–1208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

