Reduced pathology and improved behavioral performance in Alzheimer's disease mice vaccinated with HSV amplicons expressing amyloid-beta and interleukin-4
- PMID: 18388924
- PMCID: PMC2441486
- DOI: 10.1038/mt.2008.39
Reduced pathology and improved behavioral performance in Alzheimer's disease mice vaccinated with HSV amplicons expressing amyloid-beta and interleukin-4
Abstract
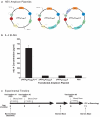
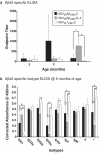
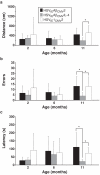
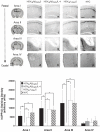
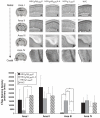
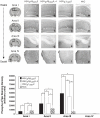
Immunotherapeutics designed to dissolve existing amyloid plaques or to interrupt amyloid-beta (Abeta) accumulation may be feasible for treatment and/or prevention of Alzheimer's disease (AD). "Shaping" the immune responses elicited against Abeta is requisite toward generating an efficacious and safe outcome; this can be achieved by minimizing the possibility of deleterious inflammatory reactions in the brain as observed in clinical testing of Abeta peptide/adjuvant-based modalities. Herpes simplex virus (HSV)-based amplicons can coexpress multiple antigens and/or immunomodulatory genes due to their large genetic size capacity, thereby facilitating antigen-specific immune response shaping. We have constructed an amplicon (HSV(IE)Abeta(CMV)IL-4) that co-delivers Abeta(1-42) with interleukin-4 (IL-4), a cytokine that promotes the generation of Th2-like T-cell responses, which are favored in the setting of AD immunotherapy. Triple-transgenic AD (3xTg-AD) mice, which progressively develop both amyloid and neurofibrillary tangle pathology, were vaccinated thrice with HSV(IE)Abeta(CMV)IL-4, or a set of control amplicon vectors. Increased Th2-related, Abeta-specific antibodies, improved learning and functioning of memory, and prevention of AD-related amyloid and tau pathological progression were observed significantly more in the HSV(IE)Abeta(CMV)IL-4 vaccinated mice as compared to the other experimental groups. Our study underscores the potential of Abeta immunotherapy for AD and highlights the potency of amplicons in facilitating the immune response modulation to a disease-relevant antigen.
Figures


Similar articles
-
Reduced Pathology and Improved Behavioral Performance in Alzheimer's Disease Mice Vaccinated With HSV Amplicons Expressing Amyloid-β and Interleukin-4.Mol Ther. 2008 May;16(5):845-853. doi: 10.1038/mt.2008.39. Epub 2016 Dec 8. Mol Ther. 2008. PMID: 28178487
-
HSV amplicon-mediated Abeta vaccination in Tg2576 mice: differential antigen-specific immune responses.Neurobiol Aging. 2005 Apr;26(4):393-407. doi: 10.1016/j.neurobiolaging.2004.04.006. Neurobiol Aging. 2005. PMID: 15653168
-
Gene vaccination to bias the immune response to amyloid-beta peptide as therapy for Alzheimer disease.Arch Neurol. 2004 Dec;61(12):1859-64. doi: 10.1001/archneur.61.12.1859. Arch Neurol. 2004. PMID: 15596606 Free PMC article.
-
Targeting beta-amyloid pathology in Alzheimer's disease with Abeta immunotherapy.Neurotherapeutics. 2008 Jul;5(3):415-20. doi: 10.1016/j.nurt.2008.05.013. Neurotherapeutics. 2008. PMID: 18625453 Free PMC article. Review.
-
[Immunotherapy of Alzheimer's disease. Results of experimental investigations and treatment of perspectives].Fortschr Neurol Psychiatr. 2004 Apr;72(4):204-19. doi: 10.1055/s-2004-818390. Fortschr Neurol Psychiatr. 2004. PMID: 15095177 Review. German.
Cited by
-
Improved behavioral response as a valid biomarker for drug screening program in transgenic rodent models of tauopathies.Cell Mol Neurobiol. 2009 Sep;29(6-7):937-44. doi: 10.1007/s10571-009-9378-2. Epub 2009 Mar 13. Cell Mol Neurobiol. 2009. PMID: 19283467 Free PMC article. Review.
-
Immune-directed gene therapeutic development for Alzheimer's, prion, and Parkinson's diseases.J Neuroimmune Pharmacol. 2009 Sep;4(3):298-308. doi: 10.1007/s11481-008-9133-3. Epub 2008 Oct 18. J Neuroimmune Pharmacol. 2009. PMID: 18931916 Free PMC article. Review.
-
Systemic vaccination with anti-oligomeric monoclonal antibodies improves cognitive function by reducing Aβ deposition and tau pathology in 3xTg-AD mice.J Neurochem. 2013 Aug;126(4):473-82. doi: 10.1111/jnc.12305. Epub 2013 Jun 16. J Neurochem. 2013. PMID: 23672786 Free PMC article.
-
Silibinin ameliorates Aβ25-35-induced memory deficits in rats by modulating autophagy and attenuating neuroinflammation as well as oxidative stress.Neurochem Res. 2017 Apr;42(4):1073-1083. doi: 10.1007/s11064-016-2141-4. Epub 2016 Dec 22. Neurochem Res. 2017. PMID: 28004303
-
Generating differentially targeted amyloid-beta specific intrabodies as a passive vaccination strategy for Alzheimer's disease.Mol Ther. 2009 Dec;17(12):2031-40. doi: 10.1038/mt.2009.174. Epub 2009 Jul 28. Mol Ther. 2009. PMID: 19638957 Free PMC article.
References
-
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. - PubMed
-
- Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. - PubMed
-
- Tariot PN, Federoff HJ. Current treatment for Alzheimer disease and future prospects. Alzheimer Dis Assoc Disord. 2003;17(Suppl 4):S105–113. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

