Deciphering interplay between Salmonella invasion effectors
- PMID: 18389058
- PMCID: PMC2268969
- DOI: 10.1371/journal.ppat.1000037
Deciphering interplay between Salmonella invasion effectors
Abstract
Bacterial pathogens have evolved a specialized type III secretion system (T3SS) to translocate virulence effector proteins directly into eukaryotic target cells. Salmonellae deploy effectors that trigger localized actin reorganization to force their own entry into non-phagocytic host cells. Six effectors (SipC, SipA, SopE/2, SopB, SptP) can individually manipulate actin dynamics at the plasma membrane, which acts as a 'signaling hub' during Salmonella invasion. The extent of crosstalk between these spatially coincident effectors remains unknown. Here we describe trans and cisbinary entry effector interplay (BENEFIT) screens that systematically examine functional associations between effectors following their delivery into the host cell. The results reveal extensive ordered synergistic and antagonistic relationships and their relative potency, and illuminate an unexpectedly sophisticated signaling network evolved through longstanding pathogen-host interaction.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




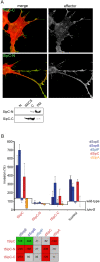

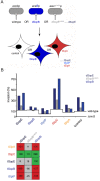

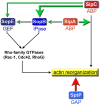
References
-
- Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. - PubMed
-
- Finlay BB, Falkow S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J Infect Dis. 1990;162:1096–1106. - PubMed
-
- Patel JC, Galan JE. Manipulation of the host actin cytoskeleton by Salmonella–all in the name of entry. Curr Opin Microbiol. 2005;8:10–15. - PubMed
-
- Collazo CM, Galan JE. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol Microbiol. 1997;24:747–756. - PubMed
-
- Hayward RD, Cain RJ, McGhie EJ, Phillips N, Garner MJ, et al. Cholesterol binding by the bacterial type III translocon is essential for virulence effector delivery into mammalian cells. Mol Microbiol. 2005;56:590–603. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

