Clinical, neuropathological and genotypic variability in SNCA A53T familial Parkinson's disease. Variability in familial Parkinson's disease
- PMID: 18389263
- PMCID: PMC2728685
- DOI: 10.1007/s00401-008-0372-4
Clinical, neuropathological and genotypic variability in SNCA A53T familial Parkinson's disease. Variability in familial Parkinson's disease
Abstract
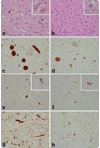
Individuals with familial Parkinson's disease (PD) due to a monogenic defect can show considerable clinical and neuropathological variability. To identify factors underlying this variability, histopathological analysis was performed in two clinically different A53T alpha-synuclein heterozygotes from Family H, a multigenerational alpha-synuclein A53T kindred. To determine whether additional genetic factors could contribute to phenotypic variability, Family H and another multigenerational A53T kindred were analyzed for parkin polymorphisms. We identified a previously described variant in parkin exon 4 associated with increased PD risk (S167N). The two A53T heterozygotes had markedly different neuropathology and different parkin genotypes: A N167 homozygote had early onset rapidly progressive disease, early dementia, myoclonus and sleep disorder, while a S167 homozygote had late onset, slowly progressive disease and late dementia. Both had brainstem, cortical, and intraneuritic Lewy bodies (LB). The N167 individual had widespread cortical neurofibrillary degeneration, while the S167 individual had only medial temporal lobe neurofibrillary degeneration. The N167 individual had severe neuronal loss in CA2 associated with Lewy neurites (LN), while the S167 individual had severe neuronal loss in CA1 associated with TDP-43 immunoreactive neuronal inclusions. These findings implicate TDP-43 in the pathology of familial PD and suggest that parkin may act as a modifier of the A53T alpha-synuclein phenotype of familial PD. Furthermore, they suggest a mechanism by which a rare genetic variant that is associated with a minor increase of PD risk in the heterozygous state may, in the homozygous state, exacerbate a disease phenotype associated with a highly penetrant dominant allele.
Figures





References
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. - PubMed
-
- Ayala YM, Pantano S, D'Ambroglio A, Buratti E, Brindisi A, Marchetti C, Romano M, Baralle FE. Human, Drosophila and C.elegans TDP-43: Nucleic acid binding properties and splicing regulatory function. J Mol Biol. 2005;348:575–588. - PubMed
-
- Baptista MJ, Cookson MR, Miller DW. Parkin and alpha-synuclein: opponent actions in the pathogenesis of Parkinson's disease. Neuroscientist. 2004;10:63–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

