Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome
- PMID: 18390493
- PMCID: PMC2364862
- DOI: 10.1136/bmj.39524.439618.25
Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome
Abstract
Objective: To investigate whether placebo effects can experimentally be separated into the response to three components-assessment and observation, a therapeutic ritual (placebo treatment), and a supportive patient-practitioner relationship-and then progressively combined to produce incremental clinical improvement in patients with irritable bowel syndrome. To assess the relative magnitude of these components.
Design: A six week single blind three arm randomised controlled trial.
Setting: Academic medical centre.
Participants: 262 adults (76% women), mean (SD) age 39 (14), diagnosed by Rome II criteria for and with a score of > or =150 on the symptom severity scale.
Interventions: For three weeks either waiting list (observation), placebo acupuncture alone ("limited"), or placebo acupuncture with a patient-practitioner relationship augmented by warmth, attention, and confidence ("augmented"). At three weeks, half of the patients were randomly assigned to continue in their originally assigned group for an additional three weeks.
Main outcome measures: Global improvement scale (range 1-7), adequate relief of symptoms, symptom severity score, and quality of life.
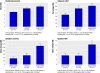
Results: At three weeks, scores on the global improvement scale were 3.8 (SD 1.0) v 4.3 (SD 1.4) v 5.0 (SD 1.3) for waiting list versus "limited" versus "augmented," respectively (P<0.001 for trend). The proportion of patients reporting adequate relief showed a similar pattern: 28% on waiting list, 44% in limited group, and 62% in augmented group (P<0.001 for trend). The same trend in response existed in symptom severity score (30 (63) v 42 (67) v 82 (89), P<0.001) and quality of life (3.6 (8.1) v 4.1 (9.4) v 9.3 (14.0), P<0.001). All pairwise comparisons between augmented and limited patient-practitioner relationship were significant: global improvement scale (P<0.001), adequate relief of symptoms (P<0.001), symptom severity score (P=0.007), quality of life (P=0.01). Results were similar at six week follow-up.
Conclusion: Factors contributing to the placebo effect can be progressively combined in a manner resembling a graded dose escalation of component parts. Non-specific effects can produce statistically and clinically significant outcomes and the patient-practitioner relationship is the most robust component.
Trial registration: Clinical Trials NCT00065403.
Figures
Comment in
-
What is the placebo worth?BMJ. 2008 May 3;336(7651):967-8. doi: 10.1136/bmj.39535.344201.BE. Epub 2008 Apr 3. BMJ. 2008. PMID: 18390494 Free PMC article.
-
Placebo effect: Helping patients feel better.BMJ. 2008 May 17;336(7653):1086-7. doi: 10.1136/bmj.39577.459549.3A. BMJ. 2008. PMID: 18483022 Free PMC article. No abstract available.
-
Placebo effect: Reconceptualising placebo.BMJ. 2008 May 17;336(7653):1086. doi: 10.1136/bmj.39577.518009.3A. BMJ. 2008. PMID: 18483023 Free PMC article. No abstract available.
-
Components of the placebo effect.Forsch Komplementmed. 2008 Aug;15(4):230-2. Forsch Komplementmed. 2008. PMID: 18798356 No abstract available.
-
Placebo acupuncture improved symptoms and quality of life in irritable bowel syndrome.Evid Based Med. 2008 Dec;13(6):180. doi: 10.1136/ebm.13.6.180. Evid Based Med. 2008. PMID: 19043038 No abstract available.
References
-
- Kaptchuk TJ. Powerful placebo: the dark side of the randomized controlled trial. Lancet 1998;351:1722-5. - PubMed
-
- Hrobjartsson A. What are the main methodological problems in the estimation of placebo effects. J Clin Epidemiol 2002;55:430-5. - PubMed
-
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006;130:1480-91. - PubMed
-
- Mitchell CM, Drossman DA. Survey of the AGA membership relating to patients with functional gastrointestinal disorders. Gastroenterology 1987;92:1282-4. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical