Structural and enzymatic analysis of MshA from Corynebacterium glutamicum: substrate-assisted catalysis
- PMID: 18390549
- PMCID: PMC2414306
- DOI: 10.1074/jbc.M801017200
Structural and enzymatic analysis of MshA from Corynebacterium glutamicum: substrate-assisted catalysis
Abstract
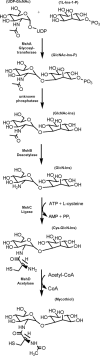
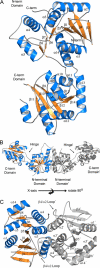
The glycosyltransferase termed MshA catalyzes the transfer of N-acetylglucosamine from UDP-N-acetylglucosamine to 1-L-myo-inositol-1-phosphate in the first committed step of mycothiol biosynthesis. The structure of MshA from Corynebacterium glutamicum was determined both in the absence of substrates and in a complex with UDP and 1-L-myo-inositol-1-phosphate. MshA belongs to the GT-B structural family whose members have a two-domain structure with both domains exhibiting a Rossman-type fold. Binding of the donor sugar to the C-terminal domain produces a 97 degrees rotational reorientation of the N-terminal domain relative to the C-terminal domain, clamping down on UDP and generating the binding site for 1-L-myo-inositol-1-phosphate. The structure highlights the residues important in binding of UDP-N-acetylglucosamine and 1-L-myo-inositol-1-phosphate. Molecular models of the ternary complex suggest a mechanism in which the beta-phosphate of the substrate, UDP-N-acetylglucosamine, promotes the nucleophilic attack of the 3-hydroxyl group of 1-L-myo-inositol-1-phosphate while at the same time promoting the cleavage of the sugar nucleotide bond.
Figures


References
-
- Demain, A. L. (1999) Appl. Microbiol. Biotechnol. 52 455-463 - PubMed
-
- Hermann, T. (2003) J. Biotechnol. 104 155-172 - PubMed
-
- Dye, C., Scheele, S., Dolin, P., Pathania, V., and Raviglione, M. C. (1999) J. Am. Med. Assoc. 282 677-686 - PubMed
-
- Britton, W. J., and Lockwood, D. N. (2004) Lancet 363 1209-1219 - PubMed
-
- Hand, C. E., and Honek, J. F. (2005) J. Nat. Prod. 68 293-308 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

