Investigating intracellular dynamics of FtsZ cytoskeleton with photoactivation single-molecule tracking
- PMID: 18390602
- PMCID: PMC2483766
- DOI: 10.1529/biophysj.108.128751
Investigating intracellular dynamics of FtsZ cytoskeleton with photoactivation single-molecule tracking
Abstract
Using photoactivatable fluorescent protein as an intracellular protein label for single-molecule tracking offers several advantages over the traditional methods. Here we demonstrate the technique of photoactivation single-molecule tracking by investigating the mobility dynamics of intracellular FtsZ protein molecules in live Escherichia coli cells. FtsZ is a prokaryotic cytoskeleton protein (a homolog of tubulin) and plays important roles in cytokinesis. We demonstrate two heterogeneous subpopulations of FtsZ molecules with distinct diffusional dynamics. The FtsZ molecules forming the Z-rings near the center of the cell were mostly stationary, consistent with the assumption that they are within polymeric filamentous structures. The rest of the FtsZ molecules, on the other hand, undergo Brownian motion spanning the whole cell length. Surprisingly, the diffusion of FtsZ is spatially restricted to helical-shaped regions, implying an energy barrier for free diffusion. Consistently, the measured mean-square displacements of FtsZ showed anomalous diffusion characteristics. These results demonstrated the feasibility and advantages of photoactivation single-molecule tracking, and suggested new levels of complexity in the prokaryotic membrane organization.
Figures
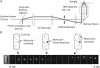
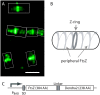
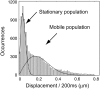
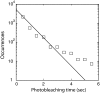



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

