Ca2+-mobility in the sarcoplasmic reticulum of ventricular myocytes is low
- PMID: 18390622
- PMCID: PMC2479570
- DOI: 10.1529/biophysj.108.130385
Ca2+-mobility in the sarcoplasmic reticulum of ventricular myocytes is low
Abstract
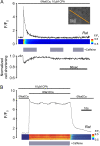
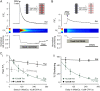
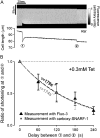
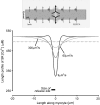
The sarcoplasmic reticulum (SR) in ventricular myocytes contains releasable Ca(2+) for activating cellular contraction. Recent measurements of intra-SR (luminal) Ca(2+) suggest a high diffusive Ca(2+)-mobility constant (D(CaSR)). This could help spatially to unify SR Ca(2+)-content ([Ca(2+)](SRT)) and standardize Ca(2+)-release throughout the cell. But measurements of localized depletions of luminal Ca(2+) (Ca(2+)-blinks), associated with local Ca(2+)-release (Ca(2+)-sparks), suggest D(CaSR) may actually be low. Here we describe a novel method for measuring D(CaSR). Using a cytoplasmic Ca(2+)-fluorophore, we estimate regional [Ca(2+)](SRT) from localized, caffeine-induced SR Ca(2+)-release. Caffeine microperfusion of one end of a guinea pig or rat myocyte diffusively empties the whole SR at a rate indicating D(CaSR) is 8-9 microm(2)/s, up to tenfold lower than previous estimates. Ignoring background SR Ca(2+)-leakage in our measurement protocol produces an artifactually high D(CaSR) (>40 microm(2)/s), which may also explain the previous high values. Diffusion-reaction modeling suggests that a low D(CaSR) would be sufficient to support local SR Ca(2+)-signaling within sarcomeres during excitation-contraction coupling. Low D(CaSR) also implies that [Ca(2+)](SRT) may readily become spatially nonuniform, particularly under pathological conditions of spatially nonuniform Ca(2+)-release. Local control of luminal Ca(2+), imposed by low D(CaSR), may complement the well-established local control of SR Ca(2+)-release by Ca(2+)-channel/ryanodine receptor couplons.
Figures



Similar articles
-
A novel mechanism of tandem activation of ryanodine receptors by cytosolic and SR luminal Ca2+ during excitation-contraction coupling in atrial myocytes.J Physiol. 2017 Jun 15;595(12):3835-3845. doi: 10.1113/JP273611. Epub 2017 Feb 1. J Physiol. 2017. PMID: 28028837 Free PMC article.
-
Cytosolic energy reserves determine the effect of glycolytic sugar phosphates on sarcoplasmic reticulum Ca2+ release in cat ventricular myocytes.J Physiol. 2006 Nov 15;577(Pt 1):281-93. doi: 10.1113/jphysiol.2006.117242. Epub 2006 Aug 31. J Physiol. 2006. PMID: 16945967 Free PMC article.
-
Regional differences in spontaneous Ca2+ spark activity and regulation in cat atrial myocytes.J Physiol. 2006 May 1;572(Pt 3):799-809. doi: 10.1113/jphysiol.2005.103267. J Physiol. 2006. PMID: 16484302 Free PMC article.
-
Calcium movements inside the sarcoplasmic reticulum of cardiac myocytes.J Mol Cell Cardiol. 2013 May;58:59-66. doi: 10.1016/j.yjmcc.2013.01.002. Epub 2013 Jan 13. J Mol Cell Cardiol. 2013. PMID: 23321551 Free PMC article. Review.
-
Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction.Annu Rev Physiol. 2014;76:107-27. doi: 10.1146/annurev-physiol-020911-153308. Epub 2013 Nov 13. Annu Rev Physiol. 2014. PMID: 24245942 Review.
Cited by
-
Three-Dimensional Model of Sub-Plasmalemmal Ca2+ Microdomains Evoked by the Interplay Between ORAI1 and InsP3 Receptors.Front Immunol. 2021 Apr 28;12:659790. doi: 10.3389/fimmu.2021.659790. eCollection 2021. Front Immunol. 2021. PMID: 33995380 Free PMC article.
-
Image-Driven Modeling of Nanoscopic Cardiac Function: Where Have We Come From, and Where Are We Going?Front Physiol. 2022 Mar 8;13:834211. doi: 10.3389/fphys.2022.834211. eCollection 2022. Front Physiol. 2022. PMID: 35356084 Free PMC article. Review.
-
Oxidative stress and ca(2+) release events in mouse cardiomyocytes.Biophys J. 2014 Dec 16;107(12):2815-2827. doi: 10.1016/j.bpj.2014.10.054. Biophys J. 2014. Retraction in: Biophys J. 2015 Nov 3;109(9):1996. doi: 10.1016/j.bpj.2015.10.007. PMID: 25517148 Free PMC article. Retracted.
-
The intricacies of atrial calcium cycling during excitation-contraction coupling.J Gen Physiol. 2017 Sep 4;149(9):857-865. doi: 10.1085/jgp.201711809. Epub 2017 Aug 10. J Gen Physiol. 2017. PMID: 28798277 Free PMC article. Review.
-
Instabilities of the resting state in a mathematical model of calcium handling in cardiac myocytes.Math Biosci. 2012 Apr;236(2):97-107. doi: 10.1016/j.mbs.2012.02.005. Epub 2012 Mar 3. Math Biosci. 2012. PMID: 22391458 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

