Inhibition of recombinant human T-type calcium channels by Delta9-tetrahydrocannabinol and cannabidiol
- PMID: 18390906
- PMCID: PMC3259625
- DOI: 10.1074/jbc.M707104200
Inhibition of recombinant human T-type calcium channels by Delta9-tetrahydrocannabinol and cannabidiol
Abstract
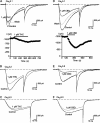
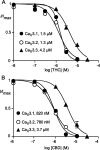
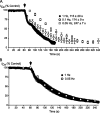
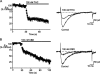
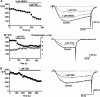
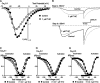
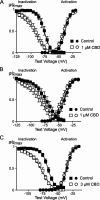
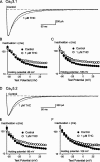
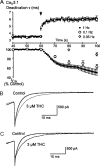
Delta(9)-Tetrahydrocannabinol (THC) and cannabidiol (CBD) are the most prevalent biologically active constituents of Cannabis sativa. THC is the prototypic cannabinoid CB1 receptor agonist and is psychoactive and analgesic. CBD is also analgesic, but it is not a CB1 receptor agonist. Low voltage-activated T-type calcium channels, encoded by the Ca(V)3 gene family, regulate the excitability of many cells, including neurons involved in nociceptive processing. We examined the effects of THC and CBD on human Ca(V)3 channels stably expressed in human embryonic kidney 293 cells and T-type channels in mouse sensory neurons using whole-cell, patch clamp recordings. At moderately hyperpolarized potentials, THC and CBD inhibited peak Ca(V)3.1 and Ca(V)3.2 currents with IC(50) values of approximately 1 mum but were less potent on Ca(V)3.3 channels. THC and CBD inhibited sensory neuron T-type channels by about 45% at 1 mum. However, in recordings made from a holding potential of -70 mV, 100 nm THC or CBD inhibited more than 50% of the peak Ca(V)3.1 current. THC and CBD produced a significant hyperpolarizing shift in the steady state inactivation potentials for each of the Ca(V)3 channels, which accounts for inhibition of channel currents. Additionally, THC caused a modest hyperpolarizing shift in the activation of Ca(V)3.1 and Ca(V)3.2. THC but not CBD slowed Ca(V)3.1 and Ca(V)3.2 deactivation and inactivation kinetics. Thus, THC and CBD inhibit Ca(V)3 channels at pharmacologically relevant concentrations. However, THC, but not CBD, may also increase the amount of calcium entry following T-type channel activation by stabilizing open states of the channel.
Figures


Similar articles
-
Modulation of Recombinant Human T-Type Calcium Channels by Δ9-Tetrahydrocannabinolic Acid In Vitro.Cannabis Cannabinoid Res. 2022 Feb;7(1):34-45. doi: 10.1089/can.2020.0134. Epub 2021 Jan 21. Cannabis Cannabinoid Res. 2022. PMID: 33998881 Free PMC article.
-
A Critical Review of the Role of the Cannabinoid Compounds Δ9-Tetrahydrocannabinol (Δ9-THC) and Cannabidiol (CBD) and their Combination in Multiple Sclerosis Treatment.Molecules. 2020 Oct 25;25(21):4930. doi: 10.3390/molecules25214930. Molecules. 2020. PMID: 33113776 Free PMC article. Review.
-
Inhibition of recombinant Ca(v)3.1 (alpha(1G)) T-type calcium channels by the antipsychotic drug clozapine.Eur J Pharmacol. 2010 Jan 25;626(2-3):123-30. doi: 10.1016/j.ejphar.2009.09.035. Epub 2009 Sep 25. Eur J Pharmacol. 2010. PMID: 19782679
-
The major phytocannabinoids, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), affect the function of CatSper calcium channels in human sperm.Hum Reprod. 2025 May 1;40(5):796-807. doi: 10.1093/humrep/deaf020. Hum Reprod. 2025. PMID: 40078063 Free PMC article.
-
The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin.Br J Pharmacol. 2008 Jan;153(2):199-215. doi: 10.1038/sj.bjp.0707442. Epub 2007 Sep 10. Br J Pharmacol. 2008. PMID: 17828291 Free PMC article. Review.
Cited by
-
Plant and fungi derived analgesic natural products targeting voltage-gated sodium and calcium channels.Channels (Austin). 2022 Dec;16(1):198-215. doi: 10.1080/19336950.2022.2103234. Channels (Austin). 2022. PMID: 36017978 Free PMC article. Review.
-
Local activation of CB1 receptors by synthetic and endogenous cannabinoids dampens burst firing mode of reticular thalamic nucleus neurons in rats under ketamine anesthesia.Exp Brain Res. 2024 Sep;242(9):2137-2157. doi: 10.1007/s00221-024-06889-6. Epub 2024 Jul 9. Exp Brain Res. 2024. PMID: 38980339
-
Cannabis for the Treatment of Epilepsy: an Update.Curr Neurol Neurosci Rep. 2018 Sep 8;18(11):73. doi: 10.1007/s11910-018-0882-y. Curr Neurol Neurosci Rep. 2018. PMID: 30194563 Review.
-
Cannabidiol Inhibits Multiple Ion Channels in Rabbit Ventricular Cardiomyocytes.Front Pharmacol. 2022 Feb 3;13:821758. doi: 10.3389/fphar.2022.821758. eCollection 2022. Front Pharmacol. 2022. PMID: 35185573 Free PMC article.
-
Direct Regulation of Hyperpolarization-Activated Cyclic-Nucleotide Gated (HCN1) Channels by Cannabinoids.Front Mol Neurosci. 2022 Apr 6;15:848540. doi: 10.3389/fnmol.2022.848540. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35465092 Free PMC article.
References
-
- xLemberger, L. (1980) Annu. Rev. Pharmacol. Toxicol. 20 151-172 - PubMed
-
- Teesson, M., Baillie, A., Lynskey, M., Manor, B., and Degenhardt, L. (2006) Drug Alcohol Depend. 81 149-155 - PubMed
-
- Gorter, R. W., Butorac, M., Cobian, E. P., and van der Sluis, W. (2005) Neurology 64 917-919 - PubMed
-
- Ware, M. A., Doyle, C. R., Woods, R., Lynch, M. E., and Clark, A. J. (2003) Pain 102 211-216 - PubMed
-
- Gross, D. W., Hamm, J., Ashworth, N. L., and Quigley, D. (2004) Neurology 62 2095-2097 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

