Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase
- PMID: 18390927
- PMCID: PMC2774791
- DOI: 10.1189/jlb.1207832
Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase
Abstract
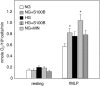
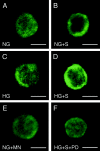
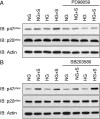
Hyperglycemia associated with diabetes mellitus results in the priming of neutrophils leading to oxidative stress that is, in part, responsible for diabetic complications. p47phox, a NADPH oxidase cytosolic subunit, is a key protein in the assembly of the NADPH oxidase leading to superoxide generation. Little is known about the priming mechanism of oxidative pathways in neutrophils of people with diabetes. In this study, the kinetics of p47phox activation was investigated by comparing neutrophils from diabetic and healthy subjects, and the mechanism of hyperglycemia-induced changes was studied by using neutrophil-like HL-60 cells as a model. In resting neutrophils from diabetic subjects, p47phox prematurely translocates to the cell membrane and preassembles with p22phox, a NADPH oxidase membrane subunit. This premature p47phox translocation and preassembly with p22phox were also observed in HL-60 cells cultured with high glucose (HG; 25 mM) and with the specific ligand for the receptor for advanced glycation end products (RAGE), S100B. Phosphorylation of ERK1/2, but not p38 MAPK, was the primary signaling pathway, as evidenced by PD98059 suppressing the translocation of p47phox in HL-60 cells incubated with HG and S100B. HL-60 cells cultured in HG and S100B exhibited a 1.8-fold increase in fMLP-induced superoxide generation compared with those cultured in normal glucose (5.5 mM). These data suggest that HG and increased AGE prime neutrophils and increase oxidative stress inducing the translocation of p47phox to the cell membrane and preassembly with p22phox by stimulating a RAGE-ERK1/2 pathway.
Figures
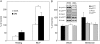



References
-
- Zimmet P., Alberti K. G., Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. - PubMed
-
- Ritz E., Orth S. R. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med. 1999;341:1127–1133. - PubMed
-
- Yamagishi S., Nakamura K., Matsui T., Takenaka K., Jinnouchi Y., Imaizumi T. Cardiovascular disease in diabetes. Mini Rev Med Chem. 2006;6:313–318. - PubMed
-
- Renard C., Van Obberghen E. Role of diabetes in atherosclerotic pathogenesis What have we learned from animal models? Diabetes Metab. 2006;32:15–29. - PubMed
-
- Pop-Busui R., Sima A., Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006;22:257–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

