Inheritance of immune polarization patterns is linked to resistance versus susceptibility to Cryptococcus neoformans in a mouse model
- PMID: 18391002
- PMCID: PMC2423067
- DOI: 10.1128/IAI.01143-07
Inheritance of immune polarization patterns is linked to resistance versus susceptibility to Cryptococcus neoformans in a mouse model
Abstract
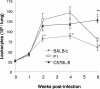
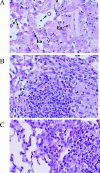
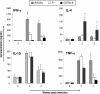
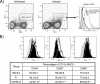
Genetic background variation between inbred strains accounts for different levels of susceptibility to Cryptococcus neoformans in the mouse infection model. To elucidate the inheritance of immunophenotypic traits and their associations with clearance outcomes during cryptococcal infection, we compared C57BL/6, BALB/c, and their first-generation hybrid, CB6F1 (F1), mice. Mice from each group were infected with C. neoformans (10(4) CFU) and analyzed at weekly intervals over a 6-week period. BALB/c mice progressively cleared the cryptococcal infection in the lungs and showed a Th1-skewed immune response: a Th1-shifted cytokine profile, modest lung pathology, and no significant elevation in the systemic immunoglobulin E (IgE) level. In contrast, C57BL/6 mice developed a chronic infection with a Th2-skewed immune response: a Th2-shifted cytokine profile, pulmonary eosinophilia, severe lung pathology, elevated serum IgE, fungemia, and cryptococcal dissemination in the central nervous system. F1 mice demonstrated intermediate resistance to C. neoformans, with a stronger resemblance to the immunophenotype of the resistant (BALB/c) mice. F1 mice also demonstrated enhanced pulmonary recruitment of lymphocytes, especially CD8(+) T cells, in comparison to both parental strains, suggesting positive heterosis. We conclude that the inheritance of traits responsible for early cytokine induction in the infected lungs and dendritic-cell maturation/activation status in draining nodes is responsible for the intermediate immune response polarization and clearance outcome observed initially in the lungs of F1 mice. The enhanced pulmonary lymphocyte recruitment could be responsible for a gradual shutdown of the undesirable Th2 arm of the immune response and subsequently improved anticryptococcal resistance in F1 mice.
Figures

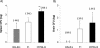

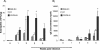

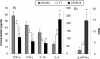
References
-
- Aguirre, K., J. Crowe, A. Haas, and J. Smith. 2004. Resistance to Cryptococcus neoformans infection in the absence of CD4+ T cells. Med. Mycol. 4215-25. - PubMed
-
- Arora, S., Y. Hernandez, J. R. Erb-Downward, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J. Immunol. 1746346-6356. - PubMed
-
- Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans, 1st ed., vol. 1. ASM Press, Washington, DC.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

