In LipL32, the major leptospiral lipoprotein, the C terminus is the primary immunogenic domain and mediates interaction with collagen IV and plasma fibronectin
- PMID: 18391007
- PMCID: PMC2423089
- DOI: 10.1128/IAI.01639-07
In LipL32, the major leptospiral lipoprotein, the C terminus is the primary immunogenic domain and mediates interaction with collagen IV and plasma fibronectin
Abstract
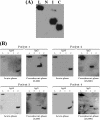
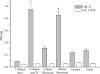
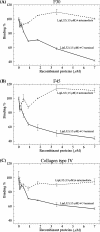
LipL32 is the major leptospiral outer membrane lipoprotein expressed during infection and is the immunodominant antigen recognized during the humoral immune response to leptospirosis in humans. In this study, we investigated novel aspects of LipL32. In order to define the immunodominant domains(s) of the molecule, subfragments corresponding to the N-terminal, intermediate, and C-terminal portions of the LipL32 gene were cloned and the proteins were expressed and purified by metal affinity chromatography. Our immunoblot results indicate that the C-terminal and intermediate domains of LipL32 are recognized by sera of patients with laboratory-confirmed leptospirosis. An immunoglobulin M response was detected exclusively against the LipL32 C-terminal fragment in both the acute and convalescent phases of illness. We also evaluated the capacity of LipL32 to interact with extracellular matrix (ECM) components. Dose-dependent, specific binding of LipL32 to collagen type IV and plasma fibronectin was observed, and the binding capacity could be attributed to the C-terminal portion of this molecule. Both heparin and gelatin could inhibit LipL32 binding to fibronectin in a concentration-dependent manner, indicating that the 30-kDa heparin-binding and 45-kDa gelatin-binding domains of fibronectin are involved in this interaction. Taken together, our results provide evidence that the LipL32 C terminus is recognized early in the course of infection and is the domain responsible for mediating interaction with ECM proteins.
Figures
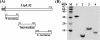



References
-
- Bharti, A. R., J. E. Nally, J. N. Ricaldi, M. A. Matthias, M. M. Diaz, M. A. Lovett, P. N. Levett, R. H. Gilman, M. R. Willig, E. Gotuzzo, and J. M. Vinetz on behalf of the Peru-United States Leptospirosis Consortium. 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 3757-771. - PubMed
-
- Bomfim, M. R. Q., A. I. Ko, and M. C. Koury. 2005. Evaluation of the recombinant LipL32 in enzyme-linked immunosorbent assay for the serodiagnosis of bovine leptospirosis. Vet. Microbiol. 10989-94. - PubMed
-
- Boonyod, D., Y. Poovorawan, P. Bhattarakosol, and C. Chirathaworn. 2005. LipL32, an outer membrane protein of Leptospira, as an antigen in a dipstick assay for diagnosis of leptospirosis. Asian Pac. J. Allergy Immunol. 23133-141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

