Immune recognition of Streptococcus pyogenes by dendritic cells
- PMID: 18391010
- PMCID: PMC2423069
- DOI: 10.1128/IAI.01680-07
Immune recognition of Streptococcus pyogenes by dendritic cells
Abstract
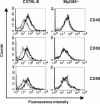
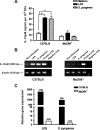
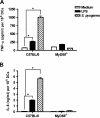
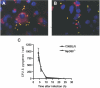
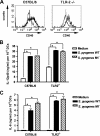
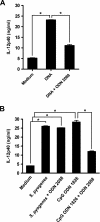
Streptococcus pyogenes is one of the most frequent human pathogens. Recent studies have identified dendritic cells (DCs) as important contributors to host defense against S. pyogenes. The objective of this study was to identify the receptors involved in immune recognition of S. pyogenes by DCs. To determine whether Toll-like receptors (TLRs) were involved in DC sensing of S. pyogenes, we evaluated the response of bone marrow-derived DCs obtained from mice deficient in MyD88, an adapter molecule used by almost all TLRs, following S. pyogenes stimulation. Despite the fact that MyD88(-/-) DCs did not differ from wild-type DCs in the ability to internalize and kill S. pyogenes, the up-regulation of maturation markers, such as CD40, CD80, and CD86, and the production of inflammatory cytokines, such as interleukin-12 (IL-12), IL-6, and tumor necrosis factor alpha, were dramatically impaired in S. pyogenes-stimulated MyD88(-/-) DCs. These results suggest that signaling through TLRs is the principal pathway by which DCs sense S. pyogenes and become activated. Surprisingly, DCs deficient in signaling through each of the TLRs reported as potential receptors for gram-positive cell components, such as TLR1, TLR2, TLR4, TLR9, and TLR2/6, were not impaired in the secretion of proinflammatory cytokines and the up-regulation of costimulatory molecules after S. pyogenes stimulation. In conclusion, our results exclude a major involvement of a single TLR or the heterodimer TLR2/6 in S. pyogenes sensing by DCs and argue for a multimodal recognition in which a combination of several different TLR-mediated signals is essential for a rapid and effective response to the pathogen.
Figures
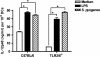
Similar articles
-
Immune receptors involved in Streptococcus suis recognition by dendritic cells.PLoS One. 2012;7(9):e44746. doi: 10.1371/journal.pone.0044746. Epub 2012 Sep 12. PLoS One. 2012. PMID: 22984550 Free PMC article.
-
Toll-like receptor 9 contributes to recognition of Mycobacterium bovis Bacillus Calmette-Guérin by Flt3-ligand generated dendritic cells.Immunobiology. 2006;211(6-8):557-65. doi: 10.1016/j.imbio.2006.05.004. Epub 2006 Jul 5. Immunobiology. 2006. PMID: 16920494
-
Group A streptococcus activates type I interferon production and MyD88-dependent signaling without involvement of TLR2, TLR4, and TLR9.J Biol Chem. 2008 Jul 18;283(29):19879-87. doi: 10.1074/jbc.M802848200. Epub 2008 May 14. J Biol Chem. 2008. PMID: 18480050 Free PMC article.
-
Toll-like receptors as adjuvant receptors.Biochim Biophys Acta. 2002 Feb 13;1589(1):1-13. doi: 10.1016/s0167-4889(01)00182-3. Biochim Biophys Acta. 2002. PMID: 11909637 Review.
-
Responses of innate immune cells to group A Streptococcus.Front Cell Infect Microbiol. 2014 Oct 2;4:140. doi: 10.3389/fcimb.2014.00140. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25325020 Free PMC article. Review.
Cited by
-
Angiotensin Converting Enzyme Inhibitors (ACEIs) Decrease the Progression of Cardiac Fibrosis in Rheumatic Heart Disease Through the Inhibition of IL-33/sST2.Front Cardiovasc Med. 2020 Jul 28;7:115. doi: 10.3389/fcvm.2020.00115. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 32850979 Free PMC article. Review.
-
Caspase-2-dependent dendritic cell death, maturation, and priming of T cells in response to Brucella abortus infection.PLoS One. 2012;7(8):e43512. doi: 10.1371/journal.pone.0043512. Epub 2012 Aug 22. PLoS One. 2012. PMID: 22927979 Free PMC article.
-
Alcohol exposure impairs myeloid dendritic cell function in rhesus macaques.Alcohol Clin Exp Res. 2009 Sep;33(9):1524-31. doi: 10.1111/j.1530-0277.2009.00980.x. Epub 2009 May 26. Alcohol Clin Exp Res. 2009. PMID: 19485975 Free PMC article.
-
IL12Rβ1: the cytokine receptor that we used to know.Cytokine. 2015 Feb;71(2):348-59. doi: 10.1016/j.cyto.2014.11.018. Epub 2014 Dec 13. Cytokine. 2015. PMID: 25516297 Free PMC article. Review.
-
Group A Streptococcus encounters with host macrophages.Future Microbiol. 2018 Jan;13(1):119-134. doi: 10.2217/fmb-2017-0142. Epub 2017 Dec 11. Future Microbiol. 2018. PMID: 29226710 Free PMC article. Review.
References
-
- Akira, S., and K. Takeda. 2004. Toll-like receptor signaling. Nat. Rev. Immunol. 4499-511. - PubMed
-
- Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2675-680. - PubMed
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. - PubMed
-
- Barton, G. M., and R. Medzhitov. 2003. Toll-like receptor signaling pathways. Science 3001524-1525. - PubMed
-
- Beutler, B., K. Hoebe, X. Du, and R. J. Ulevitch. 2003. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J. Leukoc. Biol. 74479-485. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

