MED1 phosphorylation promotes its association with mediator: implications for nuclear receptor signaling
- PMID: 18391015
- PMCID: PMC2423130
- DOI: 10.1128/MCB.02191-07
MED1 phosphorylation promotes its association with mediator: implications for nuclear receptor signaling
Abstract
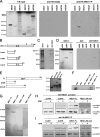
Mediator is a conserved multisubunit complex that acts as a functional interface between regulatory transcription factors and the general RNA polymerase II initiation apparatus. MED1 is a pivotal component of the complex that binds to nuclear receptors and a broad array of other gene-specific activators. Paradoxically, MED1 is found in only a fraction of the total cellular Mediator complexes, and the mechanisms regulating its binding to the core complex remain unclear. Here, we report that phosphorylation of MED1 by mitogen-activated protein kinase-extracellular signal-regulated kinase (MAPK-ERK) promotes its association with Mediator. We show that MED1 directly binds to the MED7 subunit and that ERK phosphorylation of MED1 enhances this interaction. Interestingly, we found that both thyroid and steroid hormones stimulate MED1 phosphorylation in vivo and that MED1 phosphorylation is required for its nuclear hormone receptor coactivator activity. Finally, we show that MED1 phosphorylation by ERK enhances thyroid hormone receptor-dependent transcription in vitro. Our findings suggest that ERK phosphorylation of MED1 is a regulatory mechanism that promotes MED1 association with Mediator and, as such, may facilitate a novel feed-forward action of nuclear hormones.
Figures

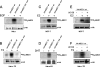
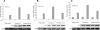




Similar articles
-
Activation of TRAP/mediator subunit TRAP220/Med1 is regulated by mitogen-activated protein kinase-dependent phosphorylation.Mol Cell Biol. 2005 Dec;25(24):10695-710. doi: 10.1128/MCB.25.24.10695-10710.2005. Mol Cell Biol. 2005. PMID: 16314496 Free PMC article.
-
Mediator subunit MED1 modulates intranuclear dynamics of the thyroid hormone receptor.J Cell Biochem. 2020 Apr;121(4):2909-2926. doi: 10.1002/jcb.29532. Epub 2019 Nov 6. J Cell Biochem. 2020. PMID: 31692077 Free PMC article.
-
Regulation of Aurora-A kinase gene expression via GABP recruitment of TRAP220/MED1.J Biol Chem. 2006 May 26;281(21):14691-9. doi: 10.1074/jbc.M600163200. Epub 2006 Mar 30. J Biol Chem. 2006. PMID: 16574658
-
Role of the mediator complex in nuclear hormone receptor signaling.Rev Physiol Biochem Pharmacol. 2006;156:23-43. doi: 10.1007/s10254-005-0002-0. Rev Physiol Biochem Pharmacol. 2006. PMID: 16634145 Review.
-
The Mediator complex in thyroid hormone receptor action.Biochim Biophys Acta. 2013 Jul;1830(7):3867-75. doi: 10.1016/j.bbagen.2012.02.012. Epub 2012 Feb 28. Biochim Biophys Acta. 2013. PMID: 22402254 Review.
Cited by
-
ERK oscillation-dependent gene expression patterns and deregulation by stress response.Chem Res Toxicol. 2014 Sep 15;27(9):1496-503. doi: 10.1021/tx500085u. Epub 2014 Aug 12. Chem Res Toxicol. 2014. PMID: 25068892 Free PMC article.
-
Cross-talk between HER2 and MED1 regulates tamoxifen resistance of human breast cancer cells.Cancer Res. 2012 Nov 1;72(21):5625-34. doi: 10.1158/0008-5472.CAN-12-1305. Epub 2012 Sep 10. Cancer Res. 2012. PMID: 22964581 Free PMC article.
-
Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors.Mol Endocrinol. 2008 Sep;22(9):2116-27. doi: 10.1210/me.2008-0059. Epub 2008 Jul 10. Mol Endocrinol. 2008. PMID: 18617595 Free PMC article.
-
Regulation of Med1 protein by overexpression of BAP1 in breast cancer cells.Mol Cell Oncol. 2024 May 2;11(1):2347827. doi: 10.1080/23723556.2024.2347827. eCollection 2024. Mol Cell Oncol. 2024. PMID: 38708315 Free PMC article.
-
ERK synchronizes embryonic cleavages in Drosophila.Dev Cell. 2024 Dec 2;59(23):3061-3071.e6. doi: 10.1016/j.devcel.2024.08.004. Epub 2024 Aug 28. Dev Cell. 2024. PMID: 39208802
References
-
- Belakavadi, M., and J. D. Fondell. 2006. Role of the mediator complex in nuclear hormone receptor signaling. Rev. Physiol. Biochem. Pharmacol. 15623-43. - PubMed
-
- Chadick, J. Z., and F. J. Asturias. 2005. Structure of eukaryotic Mediator complexes. Trends Biochem. Sci. 30264-271. - PubMed
-
- Cheskis, B. J. 2004. Regulation of cell signalling cascades by steroid hormones. J. Cell Biochem. 9320-27. - PubMed
-
- Conaway, J. W., L. Florens, S. Sato, C. Tomomori-Sato, T. J. Parmely, T. Yao, S. K. Swanson, C. A. Banks, M. P. Washburn, and R. C. Conaway. 2005. The mammalian Mediator complex. FEBS Lett. 579904-908. - PubMed
-
- Crawford, S. E., C. Qi, P. Misra, V. Stellmach, M. S. Rao, J. D. Engel, Y. Zhu, and J. K. Reddy. 2002. Defects of the heart, eye, and megakaryocytes in peroxisome proliferator activator receptor-binding protein (PBP) null embryos implicate GATA family of transcription factors. J. Biol. Chem. 2773585-3592. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
