The neogenin intracellular domain regulates gene transcription via nuclear translocation
- PMID: 18391016
- PMCID: PMC2423113
- DOI: 10.1128/MCB.02114-07
The neogenin intracellular domain regulates gene transcription via nuclear translocation
Abstract
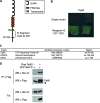
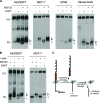
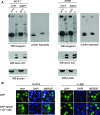
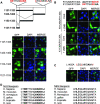
Neogenin is a multifunctional receptor implicated in axon navigation, neuronal differentiation, morphogenesis, and cell death. Very little is known about signaling downstream of neogenin. Because we found that the neogenin intracellular domain (NeICD) interacts with nuclear proteins implicated in transcription regulation, we investigated further whether neogenin signals similarly to the Notch receptor. We show here that neogenin is cleaved by gamma-secretase, an event that releases the complete NeICD. We also describe that NeICD is located at the nucleus, a feature regulated through a balance between nuclear import and export. NeICD triggers gene reporter transactivation and associates with nuclear chromatin. Direct transcriptional targets of NeICD were determined and were shown to be up-regulated in the presence of neogenin ligand. Together, we reveal here a novel aspect of neogenin signaling that relies on the direct implication of its intracellular domain in transcriptional regulation.
Figures

Similar articles
-
Protein 14-3-3sigma interacts with and favors cytoplasmic subcellular localization of the glucocorticoid receptor, acting as a negative regulator of the glucocorticoid signaling pathway.J Biol Chem. 2003 Jul 11;278(28):25651-6. doi: 10.1074/jbc.M302818200. Epub 2003 May 1. J Biol Chem. 2003. PMID: 12730237
-
Neogenin: one receptor, many functions.Int J Biochem Cell Biol. 2007;39(5):874-8. doi: 10.1016/j.biocel.2006.10.023. Epub 2006 Nov 2. Int J Biochem Cell Biol. 2007. PMID: 17137827 Review.
-
LIM-only protein 4 interacts directly with the repulsive guidance molecule A receptor Neogenin.J Neurochem. 2008 Oct;107(2):418-31. doi: 10.1111/j.1471-4159.2008.05621.x. Epub 2008 Aug 12. J Neurochem. 2008. PMID: 18702663
-
Dissection of amyloid-beta precursor protein-dependent transcriptional transactivation.J Biol Chem. 2004 Jun 4;279(23):24601-11. doi: 10.1074/jbc.M402248200. Epub 2004 Mar 24. J Biol Chem. 2004. PMID: 15044485
-
Emerging roles for neogenin and its ligands in CNS development.J Neurochem. 2008 Aug;106(4):1483-92. doi: 10.1111/j.1471-4159.2008.05485.x. Epub 2008 May 15. J Neurochem. 2008. PMID: 18485097 Review.
Cited by
-
ϒ-secretase and LARG mediate distinct RGMa activities to control appropriate layer targeting within the optic tectum.Cell Death Differ. 2016 Mar;23(3):442-53. doi: 10.1038/cdd.2015.111. Epub 2015 Aug 21. Cell Death Differ. 2016. PMID: 26292756 Free PMC article.
-
Protease regulation: the Yin and Yang of neural development and disease.Neuron. 2011 Oct 6;72(1):9-21. doi: 10.1016/j.neuron.2011.09.012. Neuron. 2011. PMID: 21982365 Free PMC article. Review.
-
RIP at the Synapse and the Role of Intracellular Domains in Neurons.Neuromolecular Med. 2020 Mar;22(1):1-24. doi: 10.1007/s12017-019-08556-4. Epub 2019 Jul 25. Neuromolecular Med. 2020. PMID: 31346933 Review.
-
Amyloid precursor protein regulates netrin-1-mediated commissural axon outgrowth.J Biol Chem. 2012 Aug 24;287(35):30014-23. doi: 10.1074/jbc.M111.324780. Epub 2012 Jul 10. J Biol Chem. 2012. PMID: 22782894 Free PMC article.
-
Receptor tyrosine kinases in the nucleus.Cold Spring Harb Perspect Biol. 2013 Oct 1;5(10):a008979. doi: 10.1101/cshperspect.a008979. Cold Spring Harb Perspect Biol. 2013. PMID: 24086039 Free PMC article. Review.
References
-
- Bray, S. J. 2006. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7678-689. - PubMed
-
- Bredesen, D. E., P. Mehlen, and S. Rabizadeh. 2005. Receptors that mediate cellular dependence. Cell Death Differ. 121031-1043. - PubMed
-
- Campbell, D. S., and C. E. Holt. 2003. Apoptotic pathway and MAPKs differentially regulate chemotropic responses of retinal growth cones. Neuron 37939-952. - PubMed
-
- Cao, X., and T. C. Südhof. 2001. A transcriptively [sic] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 293115-120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
