Population pharmacokinetics of rifampin in pulmonary tuberculosis patients, including a semimechanistic model to describe variable absorption
- PMID: 18391026
- PMCID: PMC2415769
- DOI: 10.1128/AAC.00461-07
Population pharmacokinetics of rifampin in pulmonary tuberculosis patients, including a semimechanistic model to describe variable absorption
Abstract
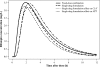
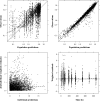
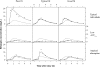
This article describes the population pharmacokinetics of rifampin in South African pulmonary tuberculosis patients. Three datasets containing 2,913 rifampin plasma concentration-time data points, collected from 261 South African pulmonary tuberculosis patients aged 18 to 72 years and weighing 28.5 to 85.5 kg and receiving regular daily treatment that included administration of rifampin (450 to 600 mg) for at least 10 days, were pooled. A compartmental pharmacokinetic model was developed using nonlinear mixed-effects modeling. Variability in the shape of the absorption curve was described using a flexible transit compartment model, in which a delay in the onset of absorption and a gradually changing absorption rate were modeled as the passage of drug through a chain of hypothetical compartments, ultimately reaching the absorption compartment. A previously described implementation was extended to allow its application to multiple-dosing data. The typical population estimate of oral clearance was 19.2 liters x h(-1), while the volume of distribution was estimated to be 53.2 liters. Interindividual variability was estimated to be 52.8% for clearance and 43.4% for volume of distribution. Interoccasional variability was estimated for CL/F (22.5%) and mean transit time during absorption (67.9%). The use of single-drug formulations was found to increase both the mean transit time (by 104%) and clearance (by 23.6%) relative to fixed-dose-combination use. A strong correlation between clearance and volume of distribution suggested substantial variability in bioavailability, which could have clinical implications, given the dependence of treatment effectiveness on exposure. The final model successfully described rifampin pharmacokinetics in the population studied and is suitable for simulation in this context.
Figures

References
-
- Acocella, G. 1978. Clinical pharmacokinetics of rifampicin. Clin. Pharmacokinet. 3:108-127. - PubMed
-
- Acocella, G., R. Mattiussi, and G. Segre. 1978. Multicompartmental analysis of serum, urine and bile concentrations of rifampicin and desacetyl-rifampicin in subjects treated for one week. Pharmacol. Res. Commun. 10:271-288. - PubMed
-
- Acocella, G., V. Pagani, M. Marchetti, G. C. Baroni, and F. B. Nicolis. 1971. Kinetic studies on rifampicin. I. Serum concentration analysis in subjects treated with different oral doses over a period of two weeks. Chemotherapy 16:356-370. - PubMed
-
- Agrawal, S., and R. Panchagnula. 2005. Implication of biopharmaceutics and pharmacokinetics of rifampicin in variable bioavailability from solid oral dosage forms. Biopharm. Drug Dispos. 26:321-334. - PubMed
-
- Bass, J. B., Jr., L. S. Farer, P. C. Hopewell, R. O'Brien, R. F. Jacobs, F. Ruben, D. E. Snider, Jr., and G. Thornton. 1994. Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and the Centers for Disease Control and Prevention. Am. J. Respir. Crit. Care Med. 149:1359-1374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

