Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice
- PMID: 18391064
- PMCID: PMC2292232
- DOI: 10.1084/jem.20072051
Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice
Abstract
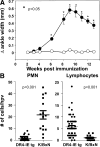
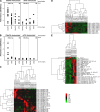
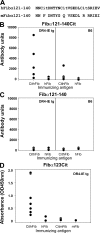
Rheumatoid arthritis (RA) is a common autoimmune disease that afflicts the synovium of diarthrodial joints. The pathogenic mechanisms inciting this disease are not fully characterized, but may involve the loss of tolerance to posttranslationally modified (citrullinated) antigens. We have demonstrated that this modification leads to a selective increase in antigenic peptide affinity for major histocompatibility complex (MHC) class II molecules that carry the RA-associated shared epitope, such as HLA-DRB1*0401 (DR4). We describe the induction of arthritis in DR4-IE transgenic (tg) mice with citrullinated fibrinogen, a protein commonly found in inflamed synovial tissue and a frequent target of autoantibodies in RA patients. The disease induced in these mice was characterized by synovial hyperplasia followed by ankylosis, but lacked a conspicuous polymorphonuclear cell infiltrate. Immunological analysis of these mice through T cell epitope scanning and antibody microarray analysis identified a unique profile of citrulline-specific reactivity that was not found in DR4-IE tg mice immunized with unmodified fibrinogen or in wild-type C57BL/6 mice immunized with citrullinated fibrinogen, two conditions where arthritis was not observed. These observations directly implicate citrullinated fibrinogen as arthritogenic in the context of RA-associated MHC class II molecules.
Figures




References
-
- Blass, S., J.M. Engel, and G.R. Burmester. 1999. The immunologic homunculus in rheumatoid arthritis. Arthritis Rheum. 42:2499–2506. - PubMed
-
- Gregersen, P.K., J. Silver, and R.J. Winchester. 1987. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 30:1205–1213. - PubMed
-
- Girbal-Neuhauser, E., J.J. Durieux, M. Arnaud, P. Dalbon, M. Sebbag, C. Vincent, M. Simon, T. Senshu, C. Masson-Bessiere, C. Jolivet-Reynaud, et al. 1999. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro)filaggrin by deimination of arginine residues. J. Immunol. 162:585–594. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

