Inhaled nitric oxide enables artificial blood transfusion without hypertension
- PMID: 18391111
- PMCID: PMC2733227
- DOI: 10.1161/CIRCULATIONAHA.107.729137
Inhaled nitric oxide enables artificial blood transfusion without hypertension
Abstract
Background: One of the major obstacles hindering the clinical development of a cell-free, hemoglobin-based oxygen carrier (HBOC) is systemic vasoconstriction.
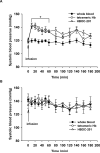
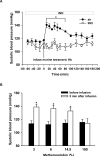
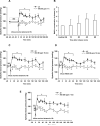
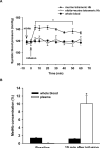
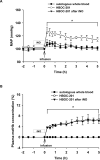
Methods and results: Experiments were performed in healthy mice and lambs by infusion of either murine tetrameric hemoglobin (0.48 g/kg) or glutaraldehyde-polymerized bovine hemoglobin (HBOC-201, 1.44 g/kg). We observed that intravenous infusion of either murine tetrameric hemoglobin or HBOC-201 induced prolonged systemic vasoconstriction in wild-type mice but not in mice congenitally deficient in endothelial nitric oxide (NO) synthase (NOS3). Treatment of wild-type mice by breathing NO at 80 ppm in air for 15 or 60 minutes or with 200 ppm NO for 7 minutes prevented the systemic hypertension induced by subsequent intravenous administration of murine tetrameric hemoglobin or HBOC-201 and did not result in conversion of plasma hemoglobin to methemoglobin. Intravenous administration of sodium nitrite (48 nmol) 5 minutes before infusion of murine tetrameric hemoglobin also prevented the development of systemic hypertension. In awake lambs, breathing NO at 80 ppm for 1 hour prevented the systemic hypertension caused by subsequent infusion of HBOC-201.
Conclusions: These findings demonstrate that HBOC can cause systemic vasoconstriction by scavenging NO produced by NOS3. Moreover, in 2 species, inhaled NO administered before the intravenous infusion of HBOC can prevent systemic vasoconstriction without causing methemoglobinemia.
Figures
Similar articles
-
Prevention of the pulmonary vasoconstrictor effects of HBOC-201 in awake lambs by continuously breathing nitric oxide.Anesthesiology. 2009 Jan;110(1):113-22. doi: 10.1097/ALN.0b013e318190bc4f. Anesthesiology. 2009. PMID: 19104178 Free PMC article.
-
Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin-based oxygen carrier.Anesthesiology. 2010 Mar;112(3):586-94. doi: 10.1097/ALN.0b013e3181cd7838. Anesthesiology. 2010. PMID: 20179495 Free PMC article.
-
Hemoglobin-based red blood cell substitutes and nitric oxide.Trends Cardiovasc Med. 2009 Apr;19(3):103-7. doi: 10.1016/j.tcm.2009.06.004. Trends Cardiovasc Med. 2009. PMID: 19679268 Free PMC article.
-
HBOC-201, hemoglobin glutamer-250 (bovine), Hemopure (Biopure Corporation).Expert Opin Biol Ther. 2008 Sep;8(9):1425-33. doi: 10.1517/14712598.8.9.1425. Expert Opin Biol Ther. 2008. PMID: 18694360 Review.
-
Systems biology of HBOC-induced vasoconstriction.Curr Drug Discov Technol. 2012 Sep;9(3):204-11. doi: 10.2174/157016312802650751. Curr Drug Discov Technol. 2012. PMID: 21726185 Free PMC article. Review.
Cited by
-
Haptoglobin or Hemopexin Therapy Prevents Acute Adverse Effects of Resuscitation After Prolonged Storage of Red Cells.Circulation. 2016 Sep 27;134(13):945-60. doi: 10.1161/CIRCULATIONAHA.115.019955. Epub 2016 Aug 11. Circulation. 2016. PMID: 27515135 Free PMC article.
-
Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs.J Clin Invest. 2009 Aug;119(8):2271-80. doi: 10.1172/JCI39115. Epub 2009 Jul 20. J Clin Invest. 2009. PMID: 19620788 Free PMC article.
-
Sodium nitrite therapy attenuates the hypertensive effects of HBOC-201 via nitrite reduction.Biochem J. 2009 Aug 27;422(3):423-32. doi: 10.1042/BJ20090735. Biochem J. 2009. PMID: 19555351 Free PMC article.
-
Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy.J Clin Invest. 2012 Apr;122(4):1444-58. doi: 10.1172/JCI59770. Epub 2012 Mar 26. J Clin Invest. 2012. PMID: 22446185 Free PMC article.
-
Inhaled nitric oxide prevents systemic and pulmonary vasoconstriction due to hemoglobin-based oxygen carrier infusion: A case report.J Crit Care. 2019 Jun;51:213-216. doi: 10.1016/j.jcrc.2018.04.008. Epub 2019 Jan 30. J Crit Care. 2019. PMID: 30709560 Free PMC article.
References
-
- Chang TMS. Blood Substitutes: Principles, Methods, Products and Clinical Trials. Vol. 1. Karger Landes Systems; Basel, New York: 1997.
-
- Chang TMS. Therapeutic applications of polymeric artificial cells. Nat Rev Drug Discovery. 2005;4:221–235. - PubMed
-
- Hare GMT, Mazer CD. Hemoglobin-based oxygen carriers. in Perioperative Transfusion Medicine. In: Spiess BD, Spence RK, Shander A, editors. 2nd edition Lippincott Williams and Wilkins; 2005. pp. 253–271.
-
- Gannon CJ, Napolitano LM. Severe anemia after gastrointestinal hemorrhage in a Jehovah's witness: new treatment strategies. Crit Care Med. 2002;30:1893–1895. - PubMed
-
- Jahr JS, Nesargi SB, Lewis K, Johnson C. Blood substitutes and oxygen therapeutics: an overview and current status. Am J Ther. 2002;9:437–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

