Role of the somatostatin system in contextual fear memory and hippocampal synaptic plasticity
- PMID: 18391186
- PMCID: PMC2327267
- DOI: 10.1101/lm.793008
Role of the somatostatin system in contextual fear memory and hippocampal synaptic plasticity
Abstract
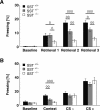
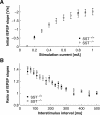
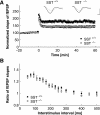
Somatostatin has been implicated in various cognitive and emotional functions, but its precise role is still poorly understood. Here, we have made use of mice with somatostatin deficiency, based upon genetic invalidation or pharmacologically induced depletion, and Pavlovian fear conditioning in order to address the contribution of the somatostatin system to associative fear memory. The results demonstrate an impairment of foreground and background contextual but not tone fear conditioning in mice with targeted ablation of the somatostatin gene. These deficits were associated with a decrease in long-term potentiation in the CA1 area of the hippocampus. Both the behavioral and the electrophysiological phenotypes were mimicked in wild-type mice through application of the somatostatin-depleting substance cysteamine prior to fear training, whereas no further deficits were observed upon application in the somatostatin null mutants. These results suggest that the somatostatin system plays a critical role in the acquisition of contextual fear memory, but not tone fear learning, and further highlights the role of hippocampal synaptic plasticity for information processing concerning contextual information.
Figures
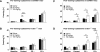
Similar articles
-
c-Rel, an NF-kappaB family transcription factor, is required for hippocampal long-term synaptic plasticity and memory formation.Learn Mem. 2008 Jul 11;15(7):539-49. doi: 10.1101/lm.866408. Print 2008 Jul. Learn Mem. 2008. PMID: 18626097 Free PMC article.
-
Depletion of dietary phytoestrogens reduces hippocampal plasticity and contextual fear memory stability in adult male mouse.Nutr Neurosci. 2021 Dec;24(12):951-962. doi: 10.1080/1028415X.2019.1698826. Epub 2019 Dec 9. Nutr Neurosci. 2021. PMID: 31814540
-
Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice.Hippocampus. 2005;15(4):418-26. doi: 10.1002/hipo.20067. Hippocampus. 2005. PMID: 15669102
-
Genes and mechanisms in the amygdala involved in the formation of fear memory.Ann N Y Acad Sci. 2003 Apr;985:92-105. doi: 10.1111/j.1749-6632.2003.tb07074.x. Ann N Y Acad Sci. 2003. PMID: 12724151 Review.
-
The amygdala, synaptic plasticity, and fear memory.Ann N Y Acad Sci. 2003 Apr;985:106-13. doi: 10.1111/j.1749-6632.2003.tb07075.x. Ann N Y Acad Sci. 2003. PMID: 12724152 Review.
Cited by
-
Decreased Numbers of Somatostatin-Expressing Neurons in the Amygdala of Subjects With Bipolar Disorder or Schizophrenia: Relationship to Circadian Rhythms.Biol Psychiatry. 2017 Mar 15;81(6):536-547. doi: 10.1016/j.biopsych.2016.04.006. Epub 2016 Apr 16. Biol Psychiatry. 2017. PMID: 27259817 Free PMC article.
-
Somatostatin: Linking Cognition and Alzheimer Disease to Therapeutic Targeting.Pharmacol Rev. 2024 Oct 16;76(6):1291-1325. doi: 10.1124/pharmrev.124.001117. Pharmacol Rev. 2024. PMID: 39013601 Free PMC article. Review.
-
Deletion of PLCγ1 in GABAergic neurons increases seizure susceptibility in aged mice.Sci Rep. 2019 Nov 28;9(1):17761. doi: 10.1038/s41598-019-54477-4. Sci Rep. 2019. PMID: 31780806 Free PMC article.
-
Childhood Adversities are not a Predictors of SSTR4met in Alcoholics.Transl Neurosci. 2017 Oct 29;8:127-138. doi: 10.1515/tnsci-2017-0019. eCollection 2017. Transl Neurosci. 2017. PMID: 29104801 Free PMC article.
-
Electrophysiological and Morphological Characterization of Chrna2 Cells in the Subiculum and CA1 of the Hippocampus: An Optogenetic Investigation.Front Cell Neurosci. 2018 Feb 13;12:32. doi: 10.3389/fncel.2018.00032. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29487503 Free PMC article.
References
-
- Abel T., Lattal K.M. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr. Opin. Neurobiol. 2001;11:180–187. - PubMed
-
- Anagnostaras S.G., Maren S., Sage J.R., Goodrich S., Fanselow M.S. Scopolamine and Pavlovian fear conditioning in rats: Dose-effect analysis. Neuropsychopharmacology. 1999;21:731–744. - PubMed
-
- Anagnostaras S.G., Gale G.D., Fanselow M.S. Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus. 2001;11:8–17. - PubMed
-
- Bakhit C., Swerdlow N. Behavioral changes following central injection of cysteamine in rats. Brain Res. 1986;365:159–163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
