Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease
- PMID: 18391196
- PMCID: PMC2311361
- DOI: 10.1073/pnas.0801677105
Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease
Abstract
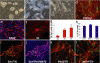
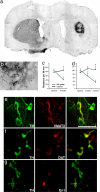
The long-term goal of nuclear transfer or alternative reprogramming approaches is to create patient-specific donor cells for transplantation therapy, avoiding immunorejection, a major complication in current transplantation medicine. It was recently shown that the four transcription factors Oct4, Sox2, Klf4, and c-Myc induce pluripotency in mouse fibroblasts. However, the therapeutic potential of induced pluripotent stem (iPS) cells for neural cell replacement strategies remained unexplored. Here, we show that iPS cells can be efficiently differentiated into neural precursor cells, giving rise to neuronal and glial cell types in culture. Upon transplantation into the fetal mouse brain, the cells migrate into various brain regions and differentiate into glia and neurons, including glutamatergic, GABAergic, and catecholaminergic subtypes. Electrophysiological recordings and morphological analysis demonstrated that the grafted neurons had mature neuronal activity and were functionally integrated in the host brain. Furthermore, iPS cells were induced to differentiate into dopamine neurons of midbrain character and were able to improve behavior in a rat model of Parkinson's disease upon transplantation into the adult brain. We minimized the risk of tumor formation from the grafted cells by separating contaminating pluripotent cells and committed neural cells using fluorescence-activated cell sorting. Our results demonstrate the therapeutic potential of directly reprogrammed fibroblasts for neuronal cell replacement in the animal model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lerou PH, Daley GQ. Therapeutic potential of embryonic stem cells. Blood Rev. 2005;19:321–331. - PubMed
-
- Hochedlinger K, Jaenisch R. Nuclear transplantation, embryonic stem cells, and the potential for cell therapy. N Engl J Med. 2003;349:275–286. - PubMed
-
- Jaenisch R. Human cloning: The science and ethics of nuclear transplantation. N Engl J Med. 2004;351:2787–2791. - PubMed
-
- Weissman IL. Medicine: Politic stem cells. Nature. 2006;439:145–147. - PubMed
-
- Rideout WM, III, Hochedlinger K, Kyba M, Daley GQ, Jaenisch R. Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell. 2002;109:17–27. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

