Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts
- PMID: 18391207
- PMCID: PMC2311331
- DOI: 10.1073/pnas.0712401105
Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts
Abstract
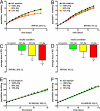
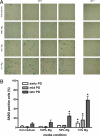
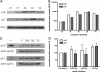
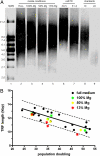
Magnesium inadequacy affects more than half of the U.S. population and is associated with increased risk for many age-related diseases, yet the underlying mechanisms are unknown. Altered cellular physiology has been demonstrated after acute exposure to severe magnesium deficiency, but few reports have addressed the consequences of long-term exposure to moderate magnesium deficiency in human cells. Therefore, IMR-90 human fibroblasts were continuously cultured in magnesium-deficient conditions to determine the long-term effects on the cells. These fibroblasts did not demonstrate differences in cellular viability or plating efficiency but did exhibit a decreased replicative lifespan in populations cultured in magnesium-deficient compared with standard media conditions, both at ambient (20% O(2)) and physiological (5% O(2)) oxygen tension. The growth rates for immortalized IMR-90 fibroblasts were not affected under the same conditions. IMR-90 fibroblast populations cultured in magnesium-deficient conditions had increased senescence-associated beta-galactosidase activity and increased p16(INK4a) and p21(WAF1) protein expression compared with cultures from standard media conditions. Telomere attrition was also accelerated in cell populations from magnesium-deficient cultures. Thus, the long-term consequence of inadequate magnesium availability in human fibroblast cultures was accelerated cellular senescence, which may be a mechanism through which chronic magnesium inadequacy could promote or exacerbate age-related disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
A connection between magnesium deficiency and aging: new insights from cellular studies.Magnes Res. 2008 Jun;21(2):77-82. Magnes Res. 2008. PMID: 18705534 Free PMC article. Review.
-
Single-cell analysis of p16(INK4a) and p21(WAF1) expression suggests distinct mechanisms of senescence in normal human and Li-Fraumeni Syndrome fibroblasts.J Cell Physiol. 2010 Apr;223(1):57-67. doi: 10.1002/jcp.22002. J Cell Physiol. 2010. PMID: 20039273
-
Telomere-based proliferative lifespan barriers in Werner-syndrome fibroblasts involve both p53-dependent and p53-independent mechanisms.J Cell Sci. 2003 Apr 1;116(Pt 7):1349-57. doi: 10.1242/jcs.00331. J Cell Sci. 2003. PMID: 12615976
-
p16/cyclin-dependent kinase inhibitor 2A deficiency in human melanocyte senescence, apoptosis, and immortalization: possible implications for melanoma progression.J Natl Cancer Inst. 2003 May 21;95(10):723-32. doi: 10.1093/jnci/95.10.723. J Natl Cancer Inst. 2003. PMID: 12759390
-
Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes.Exp Gerontol. 2000 Oct;35(8):927-45. doi: 10.1016/s0531-5565(00)00180-7. Exp Gerontol. 2000. PMID: 11121681 Review.
Cited by
-
Genome-Protecting Compounds as Potential Geroprotectors.Int J Mol Sci. 2020 Jun 24;21(12):4484. doi: 10.3390/ijms21124484. Int J Mol Sci. 2020. PMID: 32599754 Free PMC article. Review.
-
Increased Wnt/β-catenin signaling contributes to autophagy inhibition resulting from a dietary magnesium deficiency in injury-induced osteoarthritis.Arthritis Res Ther. 2022 Jul 8;24(1):165. doi: 10.1186/s13075-022-02848-0. Arthritis Res Ther. 2022. PMID: 35804467 Free PMC article.
-
Briefly bound to activate: transient binding of a second catalytic magnesium activates the structure and dynamics of CDK2 kinase for catalysis.Structure. 2011 May 11;19(5):675-90. doi: 10.1016/j.str.2011.02.016. Structure. 2011. PMID: 21565702 Free PMC article.
-
Association of magnesium depletion score with serum anti-aging protein Klotho in the middle-aged and older populations.Front Nutr. 2025 Mar 27;12:1518268. doi: 10.3389/fnut.2025.1518268. eCollection 2025. Front Nutr. 2025. PMID: 40212717 Free PMC article.
-
A connection between magnesium deficiency and aging: new insights from cellular studies.Magnes Res. 2008 Jun;21(2):77-82. Magnes Res. 2008. PMID: 18705534 Free PMC article. Review.
References
-
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: Natl Acad Press; 1997. pp. 190–249.
-
- Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. J Nutr. 2003;133:2879–2882. - PubMed
-
- Moshfegh A, Goldman J, Cleveland L. What We Eat in America, NHANES 2001–2002: Usual Nutrient Intakes from Food Compared to Dietary Reference Intakes. Washington, DC: US Department of Agriculture, Agriculture Research Service; 2005.
-
- Bowman S. Low economic status is associated with suboptimal intakes of nutritious foods by adults in the National Health and Nutrition Examination Survey 1999–2002. Nutr Res. 2007;27:513–523.
-
- Kant AK, Graubard BI. Ethnicity is an independent correlate of biomarkers of micronutrient intake and status in American adults. J Nutr. 2007;137:2456–2463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

