Towards a rational approach for heavy-atom derivative screening in protein crystallography
- PMID: 18391402
- PMCID: PMC2725783
- DOI: 10.1107/S0907444907068849
Towards a rational approach for heavy-atom derivative screening in protein crystallography
Abstract
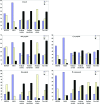
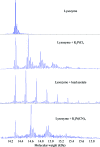
Heavy-atom derivatization is routinely used in protein structure determination and is thus of critical importance in structural biology. In order to replace the current trial-and-error heavy-atom derivative screening with a knowledge-based rational derivative-selection method, the reactivity of more than 40 heavy-atom compounds over a wide range of buffer and pH values was systematically examined using peptides which contained a single reactive amino-acid residue. Met-, Cys- and His-containing peptides were derivatized against Hg, Au and Pt compounds, while Tyr-, Glu-, Asp-, Asn- and Gln-containing peptides were assessed against Pb compounds. A total of 1668 reactive conditions were examined using mass spectrometry and were compiled into heavy-atom reactivity tables (http://sis.niaid.nih.gov/cgi-bin/heavyatom_reactivity.cgi). The results showed that heavy-atom derivatization reactions are highly linked to buffer and pH, with the most accommodating buffer being MES at pH 6. A group of 21 compounds were identified as most successful irrespective of ligand or buffer/pH conditions. To assess the applicability of the peptide heavy-atom reactivity to proteins, lysozyme crystals were derivatized with a list of peptide-reactive compounds that included both known and new compounds for lysozyme derivatization. The results showed highly consistent heavy-atom reactivities between the peptides and lysozyme.
Figures


References
-
- Blake, C. C. (1968). Adv. Protein Chem. 23, 59–120. - PubMed
-
- Blake, C. C., Fenn, R. H., North, A. C., Phillips, D. C. & Poljak, R. J. (1962). Nature (London), 196, 1173–1176. - PubMed
-
- Blake, C. C., Geisow, M. J., Swan, I. D., Rerat, C. & Rerat, B. (1974). J. Mol. Biol. 88, 1–12. - PubMed
-
- Blundell, T. L., Dodson, G. G., Dodson, E., Hodgkin, D. C. & Vijayan, M. (1971). Recent Prog. Horm. Res. 27, 1–40. - PubMed
-
- Blundell, T. L. & Jenkins, J. A. (1977). Chem. Soc. Rev. 6, 139–171.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

